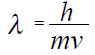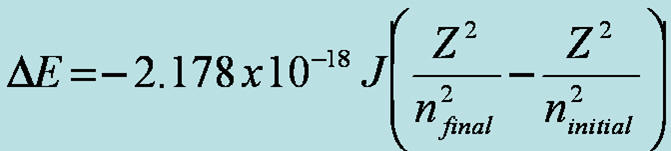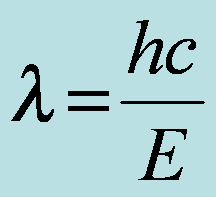AP Chemistry
AP Summer of Chemistry 2018-2019 Sign-up
- Please complete the Google form by clicking on the link and sign up for the Canvas Summer of Chemistry course.
CANVAS-LOGIN PAGE
https://canvas.instructure.com/enroll/XRAGWE
University of Texas-Quest Login
APChem92592
Turnitin.com info class ID 17736974
Turnitin Enrollement Key: IPCHEMISTRY
Email:
Mr. MacLean's School e-mail
Mr. MacLean's Home e-mail
UCR AP Readiness Flyer 2018-19.pdf --Registration
Checklist of topics for AP Chemistry.docx
| August | ||||
|
Monday |
Tuesday | Wednesday | Thursday | Friday |
|
13 Staff Development Day No School!!!
NEXT YEAR test just Chapter 1 and 2 during the first week and teach Sig Figs, Atomic Structure and Nomenclature |
14 Staff Development Day No School!!!
|
15 Target - Students will learn what the "SPIRIT" of Great Oak means. SPIRIT Day!!!
S - Scholarship Minimum Day
Create Canvas and U of T Quest Logins if you haven't already. Lab Notebook -Available at Amazon or college bookstores. Try and get it before our fist lab. Lab Notebook 2 - Here is another NEXT YEAR test just Chapter 1 and 2 during the first week and teach Sig Figs, Atomic Structure and Nomenclature |
16
Target - Students
should be able to define a theory and law and distinguish between the two. Students
will become familiar with classroom content, standards, procedures and
safety. Be
able to show how uncertainty in a measurement arises and be able to
indicate a measurement’s uncertainty by using significant figures. Expectation Sign off Sheet --if you printer is not working, please have your parent write that they read the expectations. Chapter 1 Foundations Tutorial Videos
Lab Notebook -Available at Amazon or college bookstores. Try and get it before our fist lab. Lab Notebook 2 - Here is another NEXT YEAR test just Chapter 1 and 2 during the first week and teach Sig Figs, Atomic Structure and Nomenclature |
Target -. Useful Numbers for conversions.pdf DIMENSIONAL ANALYSIS PRACTICE PROBLEMS Accident at Jefferson High Video Watch this fun video on You tube. Maybe you can do it in class for us? Flinn Safety Contract.pdf--Please get signed and turned in with expectation sheet if you haven't already
Lab Notebook -Available at Amazon or college bookstores. Try and get it before our fist lab. Lab Notebook 2 - Here is another
|
|
20 Late Start Sig Figs and Dimensional Analysis Practice Quiz Target-Students should be able to state what a mixture is and how different molecules can be separated using chromatography Separating mixtures video lessons Chemistry 203: Separation of Mixtures
|
21 Chapter 2 Target-Students should be able to state the position of protons, electrons and neutrons. State the relative mass and relative charge of protons, electrons and neutrons. Define the terms mass number (A), atomic number (Z), and isotope. State the symbol for an isotope. Explain how the isotopes of an element differ Chapter 2 Atoms, Molecules and Ions Tutorial Videos Basic Atomic Structure notes-- p+, e-, no and isotope CW Nuclear Atom Inquiry Activity Periodic Table of Ions.pdf --Useful throughout the year. HW. Click here for a list of ions you need to know. Quiz on the polyatomic ions in red tomorrow. I will announce other quizzes later in the year. Flash cards might be the best way. Here is a link to some fun games that have all the ions. HW.. Video..discovery of the proton, electron and neutron Complete list of ions and some study tools. Download it to your cell phone even. Five Experiments You Should Know.pptx
|
22 Target- Students should be able to apply IUPAC nomenclature rules to name inorganic substances and write their formula. (International Union of Pure and Applied Chemists Inorganic Nomenclature-Notes Try these nomenclature problems online--Get immediate feedback on correctness Here is more practice with nomenclature
|
23 Targets for the first exam Ch 1-2 Target--Students should know the targets for Chapter 1 and 2 Chapter 1 and 2 Study Guide on Quest Naming Acids My ride has hydrolics I ate something Icky Sprite is delicious
|
24 Target - Students should know the targets for Chapter 1 and 2 Chapter 1 and 2 Test
|
|
27 Late Start Target--Be able to calculate the atomic weight of an element given data that can be used to find the relative % of each isotope. (Mass Spec data) Determining atomic weight and % composition by mass % composition.ppt How a mass spectrometer works and finding the relative atomic weight from mass spectrometry data.ppt Another Mass Spectrometer tutorial Video showing how mass spectrometers work You Tube tutorial--Finding Average Atomic Mass Chapter 3.1-3.5 Powerpont notes
feel-good exercise hormone irisin is real. Found using a mass
spec machine.
Target--Students should be able to calculate the empirical and molecular formula of a compound when given data from a mass spectrometer. Empirical and molecular formula's Practice Practice Sheet -Not turned in.
|
28 Target---Students should be able to calculate the empirical and molecular formula of a compound in the lab |
29 Target-- Students should be able to calculate the empirical and molecular formula of a compound when given data from a mass spectrometer. Empirical and Molecular Formula POGIL -Due tomorrow
|
30 Target--Students should know the targets for Ch 1,2 and 3.1-3.5 Work on Ch 1-3.5 study guide Ch 2 Review Answer key |
31 Target--Students should know the targets for Ch 1,2 and 3.1-3.5 Chapter 3 Test
Watch this video on Youtube to see how it is really done. Then do three trials of the thisSimulation of the Hydrate Lab to calculate the % composition and then the formula of the hydrate. Use the Empirical formula RAP to help you find the formula. Please write up a short summary of the procedure and then show the calculations. The formula will look like this CuSO4 . xH2O .. x is the number of waters in the hydrate. Due Monday |
| September | ||||
| Monday | Tuesday | Wednesday | Thursday | Friday |
|
4 LABOR DAY!!! No School!!!
|
5 Target- Students should be able to apply the Law of Conservation of Mass to balance equationsGo over test results If time allows ...Balancing equations. Do problems #77,78 on page 126 in 6th edition. Canvas Balancing equations practice
|
6 Target- Students should be able to determine the mole ratio in a balanced Balancing Equations Warm-up Due Thursday Canvas balancing equations ONLINE Quiz due by tomorrow night |
7 Mass-mass problems WS Mass-mass practice problems Empirical_formula_compound.doc
|
8 Target--Students should be able to determine the limiting reactant and the reactant in excess when quantities of reacting substances are given. Given a chemical equation and the initial amounts of two or more reactants: identify the limiting reactant calculate the theoretical yield of a product calculate the amount (s) of the reactant (s) in excess remaining after the reaction is complete Peer Access Empirical Formula Lab during class-Analysis and Evaluation Empirical_formula_compound.doc
|
|
11 Target- Students should be able to determine the limiting reactant and the reactant in excess when quantities of reacting substances are given. Given a chemical equation and the initial amounts of two or more reactants: identify the limiting reactant calculate the theoretical yield of a product calculate the amount (s) of the reactant (s) in excess remaining after the reaction is complete. Good video lesson on limiting reactants Prep Lab–Limiting Reagent Lab Only Prep part 1-_We will assess Analysis and Evaluation
|
12 Target--Students should be able to determine the limiting reactant and the reactant in excess when quantities of reacting substances are given. Given a chemical equation and the initial amounts of two or more reactants: identify the limiting reactant calculate the theoretical yield of a product calculate the amount (s) of the reactant (s) in excess remaining after the reaction is complete Calculate the % yield of a reactant when there is a limiting reactant. Quest Quiz-Short Limiting Reagents due Sunday night
|
13 Target-- Students should be able to determine the limiting reactant and the reactant in excess when quantities of reacting substances are given. Given a chemical equation and the initial amounts of two or more reactants: identify the limiting reactant calculate the theoretical yield of a product calculate the amount (s) of the reactant (s) in excess remaining after the reaction is complete. Measure mass of filter paper. Limiting Reagents (reactants) Determine the %yield of PbI2 produced based upon which reactant was limiting. % yield and limiting reagent Quest Quiz due Sunday Night |
14 Target--Students should be able to determine the limiting reactant and the reactant in excess when quantities of reacting substances are given. Given a chemical equation and the initial amounts of two or more reactants: identify the limiting reactant calculate the theoretical yield of a product calculate the amount (s) of the reactant (s) in excess remaining after the reaction is complete. Work on Quest Quiz % yield and limiting reagent Quest Quiz due Sunday night
Limiting Reagent Lab Due Monday |
15 Staff Development Day
No School!!!
|
|
18 Target-- Students should be able to determine the limiting reactant and the reactant in excess when quantities of reacting substances are given. Given a chemical equation and the initial amounts of two or more reactants: identify the limiting reactant calculate the theoretical yield of a product calculate the amount (s) of the reactant (s) in excess remaining after the reaction is complete. Propogating uncertainties Absolute and % uncertainties Video Finish lab Analysis and Evaluation Lab Due Tuesday |
19 Target--Students should know the targets for chapter 1-3. Work on Prep test in class. Please print it out and bring it to class. Prep Test is due at 11 PM, not midnight. Lab Due Today Absolute and % uncertainties Video
|
20 Target--Students should know the targets for chapter 1-3. Chapter 3 Test
|
21 Target--Students should be able to define Molarity and calculate the concentration of s solution in terms of moles/L or moles/ dm3Peer Assess Limiting Reagent Lab Molarity Quest Quiz due Sunday night by midnight
|
22 Target--Students should be be able to determine if a substance is an electrolyte or non-electrolyte. Solubility Dissociation equations. -- Memorize these ASAP. Print them out for use in class.
Solubility rules and review of nomenclature --Quest Quiz due Tuesday at midnight-- -- Memorize these ASAP. Print them out for use in class. |
|
25 Late Start Students should be able to write the dissociation equation for strong electrolytes. Reaction types and electrolytes Solubility Rules and Strong and Weak Acids/ Bases -- Memorize these ASAP. Print them out for use in class. |
26 Target--Students should be able to determine the product in a precipitation reaction based upon solubility rules and write the equation for the reaction using molecular, complete ionic and net ionic equation formats. Equation Writing Solubility rules and review of nomenclature--Quest Quiz due at midnight-- Solubility Rules -- Memorize these ASAP. Print them out for use in class.
|
27 Target----Students should be able to calculate the Molarity of a solution and use molarity/volume of a solution to do stoichiometry problems Precipitation Reaction Lab-Writing net ionic equations-no solubility rules or ion sheets, just a periodic table
|
28 Target-Students should be able to write an acid-base reaction Chapter 4- reactions and solution stoichiometry Acid Base reactions Cornell University Solubility Rules Song
|
29 Target---Students should be able to determine the products of an acid base reaction and write the net ionic equation for the reaction for reactions involving both strong and weak acids with bases. Comparison of strong and weak acid-base reactions. Acid Base Equation Writing -Quest Quiz due Sunday night at midnight our time.
|
| October | ||||
| Monday | Tuesday | Wednesday | Thursday | Friday |
|
2 Target--Students should be able to determine the Net Ionic Equation of an acid base reaction. Acid/base: Net Ionic Equations Quiz Molarity Stoichiometry Problems Quest Quiz Molarity Stoichiometry due tonight at midnight.
. Lab- Prep
|
3 Target- Students should be able to calculate the concentration of Ethanoic acid in vinegar using by titrating it with a known concentration of NaOH. |
4 Target--Students should be able to determine the oxidation state/charge of elements in compounds and molecules by applying the rules for assigning oxidations states. Oxidation states -Go over the rules in section 4.9 of text Oxidation Number practice Quiz CW-Practice problems #57,59, and 61 at the end of the chapter pg 183 Oxidation States Quest Quiz due tonight at midnight our time
Target--Students should be able to balance reactions in acidic solution using the half-reaction method. Balancing reactions using the half-reaction method in acidic solution Balancing half reactions in acid--PowerPoint
|
5 Target--Students should be able to balance reactions in acidic solution using the half-reaction method. Balancing reactions using the half-reaction method in acidic solution #65,66 Balancing half reactions in acid--PowerPoint |
6 Target-- Students should be able to balance reactions in acidic and basic solution using the half-reaction method. Students should be able to define oxidation, reduction ; which substance is being oxidized and reduced; identify the oxidizing agent and reducing agent. Oxidized/Reduced substances. Oxidizing/Reducing agents. Balancing equations in basic solutions using the half reaction method. Balancing Half Ractions Quest Quiz Due Sunday night
|
|
9 Target--Students should be able to balance redox, precipitation, or acid-base reactions. Students should be able to identify which substance will ppt based upon solubility rules and apply this to writing net ionic equations for ppt reactions. Students should know which acids-bases are strong or weak and apply this to writing net ionic equations for acid-base reactions. Students should be able to identify which substance is oxidized, reduced, is the reducing agent, or the oxidizing agent in a reaction.
|
10 Target- Students should be able to determine the % Cu in Brass using Beer's Law and a colorimeter Purpose/Target of Lab Measure the absorbance of the Cu(NO3)2 solution and calculate the % of copper in the brass solution. |
11
College and Career Day |
12 Target- Students should be able to determine the % Cu in Brass using Beer's Law and a colorimeter Measure the absorbance of the Cu(NO3)2 solution and calculate the % of copper in the brass solution. Lab is due on the Tuesday. Target--Students should be able to balance redox, precipitation, or acid-base reactions. Students should be able to identify which substance will ppt based upon solubility rules and apply this to writing net ionic equations for ppt reactions. Students should know which acids-bases are strong or weak and apply this to writing net ionic equations for acid-base reactions. Students should be able to identify which substance is oxidized, reduced, is the reducing agent, or the oxidizing agent in a reaction. Bring Online Homework for work during class. Ch 4 Prep Test Online HW due at midnight Tonight
|
13 Target-- Students should be able to balance redox, precipitation, or acid-base reactions. Students should be able to identify which substance will ppt based upon solubility rules and apply this to writing net ionic equations for ppt reactions. Students should know which acids-bases are strong or weak and apply this to writing net ionic equations for acid-base reactions. Students should be able to identify which substance is oxidized, reduced, is the reducing agent, or the oxidizing agent in a reaction. Chapter 4 test
|
|
16 Target--Students should know the kinetic molecular theory postulates of gases and other properties of gases. Students should know that gases are measured with a barometer and be able to explain how a barometer works. How Cold can it get? Absolute Cold POGIL Gas Variables-S.pdf KNOW THESE Useful gas problems and relationships.pdfKinetic molecular Theory Postulates
|
17 arget -Students should be able to explain to their partner how air pressure is measured using barometers, and manometers. Students should be able to convert between units of atm, mm Hg, torr, KPa, and in of Hg. Cont...POGIL Gas Variables-S.pdf Pressure: Barometers, Manometers, and Pressure units
|
18 Lab Duearget -Students should be able to apply various gas law equations to calculate answers to various problems. Gas Laws -Boyles Law, Charles Law, and the combined gas law
Pressure measurements and conversions Online HW/Quest Quiz due tonight at midnight Gas Law Problems Online HW/Quest Quiz due Thursday night at 11 PM |
19 Target -Students should be able to apply various gas law equations to calculate answers to various problems. Gas Laws -Boyles Law, Charles Law, combined gas law and ideal gas law. PV=nRT Work on assignment during class Gas Law Problems Online HW/Quest Quiz due tonight at midnight |
20 Target - Students should be able to collect the data necessary to calculate absolute zero using Charles's Law and graph the data using Excel or other spreadsheet. Draw a best fit trend line that intersects the x-axis where the volume of the gas is zero. Use checklist when writing it up. Charles Law Lab -Assess D.P.P. and C.E. Lab Due Data Processing and Presentation Check Sheet.pdf Propagating error and uncertainty in calculations
|
|
23 Target -Students should be able to collect the data necessary to calculate absolute zero using Charles's Law and graph the data using Excel or other spreadsheet. Draw a best fit trend line that intersects the x-axis where the volume of the gas is zero. Use checklist when writing it up. Charles Law Lab -Assess D.P.P. and C.E. Data Processing and Presentation Check Sheet.pdf Propagating error and uncertainty in calculations
|
24 Target -Students should be able to calculate the pressure of a mixture of gasses using Dalton's law of partial pressure. Gas Stoichiometry / Daltons Law of Partial Pressures Practice Problems from Book
|
25 Target -Students should know the targets for all of Chapter 5 Gas Law Equation Quiz |
26 Target -Students should know the targets for all of Chapter 5 Chapter 5 Practice test Gases and their properties Practice Test.doc Answer Key Gas Stoichiometry Quest due Sunday night |
27 Target -Students should know the targets for all of Chapter 5 AP Practice FRQ Here is a great online practice midterm -self graded |
| November |
|
30 T arget- Students should know the targets for all of Chapters 1-5Chapter 1-5 study guide Quest Quiz Due Tonight at 11 PM |
31
Target--Students should know the
targets for all of Chapters 1-5 |
1
Calorimetry-S Pogil acivity.pdf-- Chapter 6 Thermochemistry-PPT notes Students should be able to define endothermic and exothermic reactions. Students should be able to calculate the molar heat of solution of a compound. Students should be able to calculate the specific heat of a metal using experimentsal data.
Prep Lab Designing a hand warmer lab --Do prep lab questions # 2 and 4 ( don't worry about heat absorbed by calorimeter) Create a data table for part b, calorimetry procedure. We will be omitting part a of lab. We will be using a stirring rod instead of a magnetic stirrer and beaker tongs instead of a heat resistant gloves. Measure temp real time to create graph and use to find heat loss like on IB Exam.
|
2 |
3 Prep-Lab-Comparing Enthalpy Changes Between Different Alcohols to Determine Fuel Efficiency.doc |
| Monday | Tuesday | Wednesday | Thursday | Friday |
|
6 Students should be able to calculate the Heat of combustion of an alcohol. Lab-Comparing Enthalpy Changes Between Different Alcohols to Determine Fuel Efficiency.doc
|
7 NGSS Target-Students should be able to calculate the amount of heat needed to change the phase of a substance or heat thesubstance using the DH=smDt equation or DH=Hf m / DH=Hv m Phase Changes and changes in Enthalpy. WS -Enthalpy Changes during phase changes practice workshee….pdf Calorimetry and Heats of Reaction Quest Quiz due Wednesday night at 1 1 PMTry these 5 problems-- Heat of combustion practice
|
8 Target- Students should be able to calculate the amount of heat needed to change the phase of a substance or heat thesubstance using the DH=smDt equation or DH=Hf m / DH=Hv m
|
9 Target- Students should be able to calculate the value and sign of the Enthalpy in reactions given data needed to do so. Heats of Formation |
10
Veteran's Day Holiday!!! No School!!!
|
|
13
|
14 Target- Students should be able to determine the sign of DS when given a formula and define what Entropy is as well as state that it is increasing in the universe. Chapter 16-Positional Entropy What is Entropy or "Why can't I get organized?" Chapter 16 powerpoint presentation http://vimeo.com/16549765 ---Thermodynamics Video Tutorial Entropy Changes Quest Quiz due tonight at midnight |
15 Target- Students should be able to apply the free energy equation to calculate spontaneity of a reaction. Students should be able to determine whether a reaction is spontaneous based upon the signs of Enthalpy, Entropy and the temperature. Entropy of the Universe D Suniverse = DSsystem + D Ssurroundings The sign of Suniverse must be ( + ) to be spontaneous. Free Energy DG=DH-TDSThe sign of G must be ( - ) to be spontaneous Gibbs Free Energy Quest Quiz due tomorrow night
|
16 Target- Students should know the targets for Chapter 16 The sign of G must be ( - ) to be spontaneous Chapter 16 Practice Test— with answers.doc Gibbs Free Energy Quest Quiz due tonight at midnight
|
17 Target- Students should know the targets for Chapter 6 Chapter 6 Practice Test with answers.doc Bring Study Guide for work in class today and Monday HW Complete all questions in the packet and be prepared for a quiz on the material after break. OChem 1.pdf - You may find a review of this material to be of substantial help. Organic Chem Nomenclature Program.zip --unzip and practice away
|
|
20
Thanksgiving Break!!! No School!!!
|
21
Thanksgiving Break!!! No School!!!
|
22
Thanksgiving Break!!! No School!!!
|
23
Thanksgiving Break!!! No School!!!
|
24
Thanksgiving Break!!! No School!!!
|
|
27 Target- Students should be able to apply the free energy equation to calculate spontaneity of a reaction. Students should be able to determine whether a reaction is spontaneous based upon the signs of Enthalpy, Entropy and the temperature. Work on Study Guide Ch 6 and 16 Study Guide Online HW Due tonight at midnight |
28 Target- Students should be able to apply the free energy equation to calculate spontaneity of a reaction. Students should be able to determine whether a reaction is spontaneous based upon the signs of Enthalpy, Entropy and the temperature. Chapter 6 and 16 Test--Test too long-shorten by 4 ?'s |
29 Target- Go over Chapter 6 and 16 Test. Apply the equation c= f x lambda Begin Chapter 7--Atomic Structure c= f x lambda chap07notes.pdf --Outline Waves, energy, and electrons online HW due Monday at midnight |
30 Target- Students should be able to calculate a DeBroglie Wavelength. Is the electron a particle or a wave? Watch the Down the Rabbit Hole video. Hey, What's your Wavelength? :-)
|
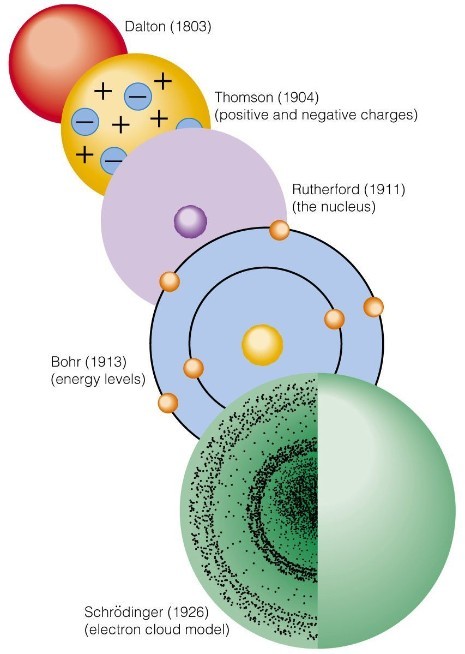
1 Line Spectra tutorial movie for Hydrogen
z=1 for Hydrogen, n is the energy level the electron is found before (initial) and after (final) the move. Waves, energy, and electrons online HW due Monday night at midnight |
| December | ||||
| Monday | Tuesday | Wednesday | Thursday | Friday |
|
4 Target- Students should be able to calculate the wavelength of EMR produced by an electron when it drops energy levels in an atom using the Rydberg equation.Line Spectra tutorial movie for Hydrogen
z=1 for Hydrogen, n is the energy level the electron is found before (initial) and after (final) the move. Nanosolar Thin Film Technology--How it works CIGS copper indium gallium (di)selenide. IR absorbance of greenhouse gases Waves, energy, and electrons online HW due Tonight at midnight |
5 Target -Students should be able to identify a Cation based on the color that it burns. FlameTest Lab.pdf |
6 Target- Students should be able to describe PES and what it is used for. Photoelectron Spectroscopy (PES) PhotoElectronSpectPOGILAct07.pdf Homework--finish up
|
7 Target- Students should be able to write electron configurations for atoms both long hand and using the Noble gas short-cut. Wave Mechanical Model of the atom
electron config filling order movie |
8 Target- Students should be able to determine the appropriate 4 quantum numbers for a particular atom, write electron configurations for atoms both long hand and using the Noble gas short-cut, draw an orbital diagram for an element, calculate the wavelength of EMR produced by an electron when it drops energy levels in an atom using the Rydberg equation, calculate a DeBroglie Wavelength, apply the equation c= f x lambda, and identify a substances cation based on the color of light produced when it burns. Chapter 7 Quiz/Test-No trends |
|
11 Late start Target- Students should be able to state the trends of effective nuclear charge, atomic radius, ionization energy and electronegativity as you move across a period and down a group of the periodic table. Uncle Zeff, electronegativity, |
12 Target -Students should be able to identify the type of bond formed between two elements based upon its difference in electronegativity. Chapter 8-Bonding Concepts.ppt Quest Quiz electronegativity and bond types Due tonight
|
13 Target-Students should be able to state the trends of effective nuclear charge, atomic radius, ionization energy and electronegativity as you move across a period and down a group of the periodic table. Uncle Zeff, electronegativity, ionization energy, etc... (periodicity) Zeff diagram scanned from old text Effective Nuclear Charge Movie atomic radii tutorial movie Summary of some periodic trends Periodic Trends Quest Quiz Due tonight at 11 PM Ion and atom size flash
|
14 Target-Students should be able to draw the correct Lewis Structure given the formula or name of an atom, ionic compound or molecule. Lewis Structures Lewis Structure Quest Quiz due Thursday night at 11 PM |
15 Target-Students should be able to identify the electron and molecular geometry of molecules using the valence shell electron pair repulsion model. VSEPR Lab-print and bring to class. HW. Hybridization and VSEPR Quest Quiz due Tuesday night at 11 PM |
|
18 Target-Students should be able to identify the electron and molecular geometry of molecules using the valence shell electron pair repulsion model. VSEPR Lab-print and bring to class. HW. Hybridization and VSEPR Quest Quiz due Tuesday night at 11 PM
|
19 Target- Students should be able to identify the type of hybridization that occurs in each molecular geometry and determine the number of sigma and pi bonds in a molecule. Hybridization types Sigma and Pi bonds Chapter 9 covanlent bonding orbitals.pdf chap 09 outline notes.pdf--only hybridization type was covered. HW. Hybridization and VSEPR Quest Quiz due tonight at 11 PM |
20 Target- Students should know EVERYTHING!! Review Day
|
21 Finals Per. 1, 2, 3 Minimum Day All Students O-Chem 2 Functional Groups--Do this Organic Chem Packet. Be prepared to work with these as soon as we come back.
AP Students HW Princeton Review Do the practice test 1 pg 13-22 # 3,4,14,15,19,21,22,24,25,26,27,28,29,30, 32,33,35,36,37,38,39,41,42,43,44,51,52,57,59,60 Grade it and return with the graded test. Work must be shown to earn credit |
22 Finals Per. 4, 5, 6 Minimum Day End of 1st Semester All Students O-Chem 2 Functional Groups--Do this Organic Chem Packet. Be prepared to work with these as soon as we come back.
AP Students HW Princeton Review Do the practice test 1 pg 13-22 # 3,4,14,15,19,20,21,22,24,25,26,27,28,29,30, 32,33,35,36,37,38,39,41,42,43,44,51,52,57,59,60 Grade it and return with the graded test. Work must be shown to earn credit |
|
19 Christmas Break!!! No School!!!
AP Students HW Princeton Review Do the practice test 1 pg 13-22 # 3,4,14,15,19,21,22,24,25,26,27,28,29,30, 32,33,35,36,37,38,39,41,42,43,44,51,52,57,59,60 Grade it and return with the graded test. Work must be shown to earn credit
|
20 Christmas Break!!! No School!!! |
21 Christmas Break!!! No School!!! |
22 Christmas Break!!! No School!!! |
23
AP Students HW Princeton Review Do the practice test 1 pg 13-22 # 3,4,14,15,19,21,22,24,25,26,27,28,29,30, 32,33,35,36,37,38,39,41,42,43,44,51,52,57,59,60 Grade it and return with the graded test. Work must be shown to earn credit Christmas Break!!! No School!!! |
|
26 Christmas Break!!! No School!!! |
27 Christmas Break!!! No School!!! |
28 Christmas Break!!! No School!!! |
29 Christmas Break!!! No School!!! |
30 Happy New Year! No School!!! |
|
January |
||||||||||||||||||
| Monday | Tuesday | Wednesday | Thursday | Friday | ||||||||||||||
|
8 Staff Development Day No School!
AP Students HW Princeton Review Do the practice test 1 pg 13-22 # 3,4,14,15,19,21,22,24,25,26, 27,28,29,30,32,33,35,36,37, 38,39,41,42,43,44,51, 52,57,59,60 Grade it and return with the graded test. Work must be shown to earn credit
|
9 Target- Students should be able to identify the type of hybridization that occurs in each molecular geometry and determine the number of sigma and pi bonds in a molecule. Hybridization types Sigma and Pi bonds Chapter 9 covanlent bonding orbitals.pdf chap 09 outline notes.pdf--only hybridization type was covered. HW. Hybridization and VSEPR Quest Quiz due tonight at midnight
IB Students will be meeting each day during Intervention for the next week |
10 Target- Students should know how to calculate the Change in Enthalpy(DH) using the bond energies provided in a table (DH = bonds broken - bonds formed.) and determine the relative length of bonds. Single bonds are longer than double bonds which are longer than triple bonds. Calculating change in Enthalpy. Bond Energy and Bond Strength Quest Quiz due tonight Bring Lab Notebook tomorrow IB Students will be meeting each day during Intervention for the next week.
|
11 Target -Students should be able to determine the type of intermolecular force of attraction between molecules Science News/Graphene.pdf--------Check this out. Looks pretty cool. Network covalent bonding. Graphene--The strongest known material. Cool Graphene Application Video by Samsung Intermolecular forces Intermolecular Forces Tutorial water balloon 1 water balloon 2 water balloon 3 Chapter 10 (solids and liquids) Hydrogen Bonding in Water Tutorial HW. Ch. 10 # 35-37 In class Edition HW. Ch. 10 # 29, 30, 31 7th Edition |
12 Target- Students should be able to draw and name a molecule from each of the different types of functional groups in the O Chem packet 2. Build Organic Molecules-Build and draw an alcohol, ether, aldehyde, ketone, organic acid, ester, amine, and amide that contain at least a 4 carbons. After you draw and name each molecule, identify the electron geometry and hybridization types of the different atoms that are found on the molecule. Functional Groups Nomenclature Chart.pdf Isomer Construction Set-Try making all the structural isomers for the first three alkanes given.IB Chem Students --- Please watch this video about methods for administering medications. Click here for videoIf you are having problems watching this movie, you can download VLC Player and it will work. Dowload VLC Click Here Quiz- Organic Functional Groups/Packet 2 Functional Groups Nomenclature Chart.pdf
|
||||||||||||||
|
15 Martin Luther King Jr. Day No School!!!
|
16 Target- Students should be able to calculate the enthalpy change that occurs during phase changes. Be able to apply Intermolecular Force concepts to properties of substances like boiling point, viscosity, vapor pressure and solubility. Vapor Pressure, Boiling Points and Phase Changes Change in Enthalpy during phase changes Quest Quiz due tomorrow night at Midnight Scientific notation is put into online hw answers as follows: 5.6 x 10-5 is entered as +5.6e-5, where “e” means “times 10 to the . . .” 6.022 x 1023 becomes +6.022e23).
|
17 Target-Students should know the difference in properties of the various types of solids.
AP POGIL Types of Solids - S.pdf Check this out!!!!! Science News/Glass Spray.pdf A major disruptor is coming. Video- Allotropes of carbon Boring, but good. Required for IB students Video-How its Made: Synthetic Diamonds. Video-Carbon Emissions & Ocean Acidification
|
18 Target--Students should know the targets for chapter 10. Work on Ch 8-10 study guide Chapter 8-10 Study Guide Quist Quiz due tonight at midnight
|
19 Target- Students should know the targets for Chapter 8-10 Chapter 8-10 Test
IB Students Aspirin Screen Experiment --Due Tuesday
|
||||||||||||||
|
22 Go Over Test Results Target- Students should be able to determine how the reaction rate of a reaction changes over time.
Kinetics/ Reactions Rates /rate laws
|
23 Target- Students should be able to determine how the reaction rate of a reaction changes over time.
Kinetics/ Reactions Rates /rate laws
|
24 Target- Students should be able to determine the rate law of a reaction using the method of initial rates. Warm-up #17 pg 598
Method of Initial RatesRate Laws-method of initial rates.pdf
|
25 Target-Students should be able to determine how the reaction rate of a reaction changes over time as well as calculate its initial rate. Work on online Quest Quiz during class. Need Chromecart Rate Laws Quest Quiz due tonight at midnight. |
26 Target-Students should be able to draw a potential energy diagram that shows the activation energy, activated complex and change in energy. Students should know what factors affect the rate of a reaction and how they will affect the rate. Examples include...temperature, concentration, use of catalyst, pressure, stirring, etc.... Collision Model--Factors that affect rates of reactions Potential Energy Diagrams IB-AP Chemistry/Chapter 12 and 13/Potential Energy Diagram ws.pdf - IB-AP Chemistry/Chapter 12 and 13/Potential energy diagram ws key.pdf #6--The top of the hump is too high on my sketch. It should not go above 450 kJ/mol. Factors that Effect Reaction Rates Tutorial Video Activation Energy and Catalysts Tutorial Video |
||||||||||||||
|
29 Target- Students should be able to determine a mechanism of a reaction given the rate law and the overall reaction. Students should know that in order for a mechanism to be considered, the steps must add to the overall reaction and the rate determining step must match the rate law. Kinetics/ Mechanisms Reaction Mechanisms Summary.PDF IB-AP Chemistry/Chapter 12 and 13/ap ch 12_practest.doc Go over mechanisms on PowerPoint New Catalyst Produces Hydrogen from Water
This might be of help. Intermediates can't be in the rate law. |
30 Target- Students should be able to determine a mechanism of a reaction given the rate law and the overall reaction. Students should know that in order for a mechanism to be considered, the steps must add to the overall reaction and the rate determining step must match the rate law. Students should know how to find the order of the reaction from graphing the data. Kinetics/ Mechanisms Reaction Mechanisms Summary.PDF Go over mechanisms on PowerPoint New Catalyst Produces Hydrogen from Water Kinetics/mechanism Quest Quiz due tonight at midnight This might be of help. Intermediates can't be in the rate law. Reaction Mechanisms Summary.PDF
|
31 Target- . |
1
Students should know equilibrium is the state where the rate of the forward reaction is equil to the rate of the reverse reaction. At these conditions, the concentrations of the reactants and products remain constant with time once equilibrium has been established at a constant temperature. Students should be able to calculate the value of K. Chapter 13 - Chemical EquilibriumNotes.ppt General Equilibrium.pdf --Notes --with practice problems.
Equilibrium Quest Quiz due tonight at midnight
Students should be able to calculate the Kp or the Kc when given the other. New Catalyst Produces Hydrogen from Water Kp relationships Reaction Quotient-- Equilibrium Calculations ICE Tables
|
2 Target- Students should be able to calculate the K, [ ], and direction a reaction will shift in a reaction.Ice tables practice with Kp and Kc Bookwork-CW 25, 31abc, 38abc, 45,47 -old text Need help? |
||||||||||||||