Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
1.
|
A volume of 1 cubic centimeter is equivalent to
a. | 1 milliliter. | c. | 1 liter. | b. | 1 gram. | d. | 10–1 cubic
decimeters. |
|
|
|
2.
|
Which of the following observations is quantitative?
a. | The liquid turns blue litmus paper red. | c. | The liquid tastes
bitter. | b. | The liquid boils at 100ºC. | d. | The liquid is
cloudy. |
|
|
|
3.
|
Quantitative observations are recorded using
a. | numerical information. | c. | non-numerical information. | b. | a
control. | d. | a
system. |
|
|
|
4.
|
Qualitative observations are recorded using
a. | numerical information. | c. | non-numerical information. | b. | a
control. | d. | a
system. |
|
|
|
5.
|
The symbol mm represents
a. | micrometer. | c. | milliliter. | b. | millimeter. | d. | meter. |
|
|
|
6.
|
The quantity of matter per unit volume is
a. | mass. | c. | inertia. | b. | weight. | d. | density. |
|
|
|
7.
|
The liter is equivilent to
a. | 1000 m3. | c. | 1000 g3. | b. | 1000 cm3. | d. | 1000
c3. |
|
|
|
8.
|
The metric unit for mass is the
a. | slug. | c. | meter. | b. | cubic centimeter. | d. | kilogram. |
|
|
|
9.
|
The symbol that represents the measured unit for volume is
|
|
|
10.
|
All of the following are SI units for density EXCEPT
a. | kg/m3. | c. | g/cm3. | b. | g/mL. | d. | g/m2. |
|
|
|
11.
|
Which statement about density is true?
a. | Two samples of a pure substance may have different densities. | b. | Density is a
chemical property. | c. | Density is a physical
property. | d. | The density of a sample depends on its location on
Earth. |
|
|
|
12.
|
The density of pure diamond is 3.5 g/cm3. The mass of a diamond is
0.25 g. Find its volume.
a. | 0.071 cm3 | c. | 3.5 cm3 | b. | 0.875 cm3 | d. | 14
cm3 |
|
|
|
13.
|
What is the density of 37.72 g of matter whose volume is 6.80
cm3?
a. | 0.18 g/cm3 | c. | 30.92 g/cm3 | b. | 5.55
g/cm3 | d. | 256.4
g/cm3 |
|
|
|
14.
|
100 milliliters is equivalent to
a. | 1 hectoliter. | c. | 1 centiliter. | b. | 1 microliter. | d. | 1 deciliter. |
|
|
|
15.
|
To two significant figures, the measurement 0.0255 g should be reported
as
a. | 0.02 g. | c. | 0.026 g. | b. | 0.025 g. | d. | 2.5 ´
102 g. |
|
|
|
16.
|
The number of significant figures in the measurement 0.000305 kg is
|
|
|
17.
|
The measurement that has been expressed to three significant figures is
a. | 0.052 g. | c. | 3.065 g. | b. | 0.202 g. | d. | 5000 g. |
|
|
|
18.
|
The measurement that has only nonsignificant zeros is
a. | 0.0037 mL. | c. | 400. mL. | b. | 60.0 mL. | d. | 506 mL. |
|
|
|
19.
|
The number that has five significant figures is
a. | 23 410 | c. | 0.01783 | b. | 0.00652 | d. | 10.292 |
|
|
|
20.
|
The product of 13 cm and 5.7 cm is correctly reported as
a. | 74 cm2. | c. | 74.1 cm2. | b. | 74.0 cm2. | d. | 75
cm2. |
|
|
|
21.
|
Round 1.245633501 ´ 108 to four
significant figures.
a. | 1246 | c. | 1.246 x 108 | b. | 1.245 x 108 | d. | 1.246 x
104 |
|
|
|
22.
|
How many significant digits should be shown in the product of 1.6 cm and 2.4
cm?
|
|
|
23.
|
The speed of light is 300,000 km/s. In scientific notation, this speed is
a. | 3 ´ 105
km/s. | c. | 3.0 ´ 106
km/s. | b. | 3.00 ´ 105
km/s. | d. | 3.00
´ 106 km/s. |
|
|
|
24.
|
The average distance between the Earth and the moon is 386,000 km. Expressed in
scientific notation, this distance is
a. | 386 ´ 103 km. | c. | 3.8 ´ 105 km. | b. | 38 ´
104 km. | d. | 3.86
´ 105 km. |
|
|
|
25.
|
An analytical balance can measure mass to the nearest 1/10,000 of a gram, 0.0001
g. In scientific notation, the accuracy of the balance would be expressed as
a. | 1.0 x 10–3 g. | c. | 1 x 104
g. | b. | 1 x 103 g. | d. | 1 x 10–4 g. |
|
|
|
26.
|
The result of dividing 107 by 10–3 is
a. | 10–4. | c. | 104. | b. | 102.5. | d. | 1010. |
|
|
|
27.
|
The expression of 5008 km in scientific notation is ____.
|
|
|
28.
|
How many significant figures are in the measurement 0.0034 kg?
a. | two | c. | five | b. | four | d. | This cannot be
determined. |
|
|
|
29.
|
How many significant figures are in the measurement 40,500 mg?
|
|
|
30.
|
How many significant figures are in the measurement 811.40 grams?
|
|
|
31.
|
Express the sum of 1111 km and 222 km using the correct number of significant
digits.
a. | 1300 km | c. | 1333 km | b. | 1330 km | d. | 1333.0 km |
|
|
|
32.
|
What is the measurement 1042 L rounded off to two significant digits?
a. | 1.0 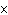 10 10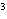 L L | c. | 1050
L | b. | 1040 L | d. | 1.1
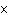 10 10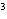 L L |
|
|
|
33.
|
What is the temperature of absolute zero measured in 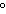 C? a. | –373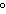 C C | c. | –173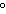 C C | b. | –273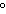 C C | d. | –73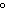 C C |
|
|
|
34.
|
Which temperature scale has no negative temperatures?
a. | Celsius | c. | Joule | b. | Fahrenheit | d. | Kelvin |
|
|
|
35.
|
What is the temperature –34 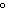 C equal to in the Kelvin
scale? a. | 139 K | c. | 239 K | b. | 207 K | d. | 339 K |
|
|
|
36.
|
If the temperature changes by 100 K, by how much does it change in 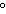 C?
|
|
|
37.
|
What is the quantity 0.0075 meters expressed in centimeters?
a. | 0.075 cm | c. | 7.5 cm | b. | 0.75 cm | d. | 70.5 cm |
|
|
|
38.
|
What is the quantity 987 milligrams expressed in grams?
a. | 0.000 987 g | c. | 9.87 g | b. | 0.987 g | d. | 98,700 g |
|
|
|
39.
|
A pint of blood would be how many mL? (1 L= 1.057
qt and 1 qt = 2 pints.)
a. | 529 mL | c. | 473 mL
| b. | 946 mL | d. | 454
mL |
|
|
|
40.
|
What is the volume of a salt crystal measuring 1.44 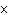 10 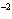 m by 2.4 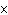 10 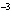 m by 7.4 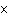
10 -1 m? a. | 2.6 x 10-5 | c. | 26 x 10-4 | b. | 2.6 x 105 | d. | 5.4 x
10-3 |
|
|
|
41.
|
What is the result of adding 2.52 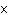 10 2 and 1.5 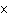 10 3?
|
|
|
42.
|
The density of gold is 19.3 g/cm3. The volume of a solid piece of
gold is 2.50 cm3. Find its mass.
a. | 8 g | c. | 48.3 g | b. | 48 g | d. | 7.72g |
|
|
|
|
|
|
43.
|
Which of the above balances would produce a measurement with the most
significant digits?
a. | Econo-Balance | c. | Balance Pro | b. | Good Balance | d. | Exacto-Balance |
|
|
|
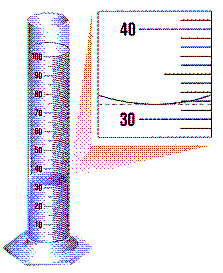
Use the following passage and the drawing to
answer the next two questions.
In a lab experiment, an unknown object’s mass and volume
were measured. The drawing shows the measurement of the object’s volume. A student went on to
measure the mass of the object. The mass was determined to be 14.7 g.
|
|
|
44.
|
The volume should be measured as :
a. | 32 mL | c. | 31.75 mL | b. | 31.8 mL | d. | 30 mL |
|
|
|
45.
|
The density of the object is :
a. | 0.462 g/mL | c. | 2.2 g/mL | b. | 0.4622 mL/g | d. | 2.16 g/mL |
|
Matching
|
|
|
Match each item with the correct statement below. a. | freezing point | d. | density | b. | Kelvin temperature scale | e. | mass | c. | Celsius temperature
scale | f. | significant
figure |
|
|
|
46.
|
known or estimated in a measurement
|
|
|
47.
|
the quantity of matter an object contains
|
|
|
48.
|
the metric scale for temperature that has no negative numbers
|
|
|
49.
|
absolute zero is measured as -273
|
|
|
50.
|
ratio of mass to volume
|