Ch. 4 Prep Test
Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
1.
|
Of the acids containing oxygen, which is true regarding those ending in -ous
compared to those ending in -ic?
a. | contains more hydrogen. | c. | contains less
oxygen. | b. | contains more oxygen. | d. | contains the same amount of oxygen. |
|
|
|
2.
|
A chemical formula includes the symbols of the elements in the compound and
subscripts that indicate
a. | the number of electrons in each element. | b. | how many atoms or
ions of each type are combined in the simplest unit. | c. | the formula mass. | d. | the charges on the
elements or ions. |
|
|
|
3.
|
Changing a subscript in a correctly written chemical formula
a. | changes the number of protons represented by the formula. | b. | changes the charges
on the other ions in the compound. | c. | changes the formula so that it no longer
represents that compound. | d. | has no effect on the
formula. |
|
|
|
4.
|
What is the formula for the compound formed by lead(II) ions and chromate
ions?
a. | PbCrO4 | c. | Pb2(CrO4)3 | b. | Pb2CrO4 | d. | Pb(CrO4)2 |
|
|
|
5.
|
What is the formula for tin(IV) chromate?
a. | Sn(CrO4)4 | c. | Sn2(CrO4)4 | b. | Sn2(CrO4)2 | d. | Sn(CrO4)2 |
|
|
|
6.
|
Name the compound CuCO3.
a. | copper(I) carbonate | c. | copper(IV) carbonate | b. | copper(III) carbonate | d. | copper(II)
carbonate |
|
|
|
7.
|
What type of ions have names ending in -ide?
a. | only cations | c. | only metal ions | b. | only anions | d. | only gaseous
ions |
|
|
|
8.
|
What is the correct name for the N 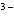
ion? a. | nitrate ion | c. | nitride ion | b. | nitrogen ion | d. | nitrite ion |
|
|
|
9.
|
When naming a transition metal ion that can have more than one common ionic
charge, the numerical value of the charge is indicated by a ____.
a. | prefix | c. | Roman numeral following the name | b. | suffix | d. | superscript after the name |
|
|
|
10.
|
Which of the following correctly provides the names and formulas of polyatomic
ions?
|
|
|
11.
|
An -ate or -ite at the end of a compound name usually indicates
that the compound contains ____.
a. | fewer electrons than protons | c. | only 1 element | b. | neutral
molecules | d. | a polyatomic
anion |
|
|
|
12.
|
Which of the following compounds contains the Mn 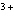 ion?
|
|
|
13.
|
Which of the following is true about the composition of ionic compounds?
a. | They are composed of anions and cations. | b. | They are composed of
anions only. | c. | They are composed of cations only. | d. | They are formed from two or more nonmetallic
elements. |
|
|
|
14.
|
Which of the following formulas represents an ionic compound?
|
|
|
15.
|
Which element, when combined with fluorine, would most likely form an ionic
compound?
a. | lithium | c. | phosphorus | b. | carbon | d. | chlorine |
|
|
|
16.
|
Which of the following correctly represents an ion pair and the ionic compound
the ions form?
|
|
|
17.
|
In which of the following is the name and formula given correctly?
a. | sodium oxide, NaO | c. | cobalt (I) chloride, CoCl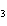 | b. | barium nitride, BaN | d. | Tin (IV) fluoride, SnF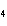 |
|
|
|
18.
|
Which of the following compounds contains the lead(II) ion?
|
|
|
19.
|
What is the correct formula for potassium sulfite?
|
|
|
20.
|
Which set of chemical name and chemical formula for the same compound is
correct?
a. | ammonium sulfite, (NH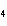 ) )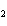 S S | c. | lithium carbonate, LiCO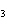 | b. | iron(III) phosphate,
FePO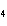 | d. | magnesium dichromate, MgCrO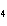 |
|
|
|
21.
|
What is the ending for the names of all binary compounds, both ionic and
Type III?
|
|
|
22.
|
Which of the following correctly shows a prefix used in naming binary molecular
compounds with its corresponding number?
a. | deca-, 10 | c. | hexa-, 8 | b. | nona-, 5 | d. | octa-, 4 |
|
|
|
23.
|
Which of the following formulas represents a Type 3 compound?
a. | ZnO | c. | SO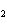 | b. | Xe | d. | BeF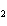 |
|
|
|
24.
|
Which of the following shows both the correct formula and correct name of an
acid?
a. | HClO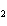 , chloric acid , chloric acid | c. | H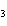 PO PO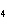 , phosphoric acid , phosphoric acid | b. | HNO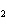 , hydronitrous acid , hydronitrous acid | d. | HI, iodic acid |
|
|
|
25.
|
What is the name of H 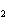 SO 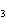 ? a. | hyposulfuric acid | c. | sulfuric acid | b. | hydrosulfuric acid | d. | sulfurous acid |
|
|
|
26.
|
What is the formula for hydrosulfuric acid?
|
|
|
27.
|
Select the correct formula for sulfur hexafluoride.
|
|
|
28.
|
What is the correct name for the compound CoCl 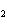 ? a. | cobalt(I) chlorate | c. | cobalt(II) chlorate | b. | cobalt(II) chloride | d. | cobalt(I)
chloride |
|
|
|
29.
|
What is the correct formula for barium chlorate?
a. | Ba(ClO3)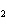 | c. | Ba(ClO)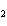 | b. | Ba(ClO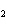 ) )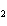 | d. | BaCl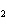 |
|
|
|
30.
|
What is the correct formula for calcium dihydrogen phosphate?
|
|
|
31.
|
Which of the following is the correct name for N 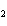 O 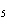 ? a. | nitrous oxide | c. | nitrogen dioxide | b. | dinitrogen pentoxide | d. | nitrate oxide |
|
|
|
32.
|
What is the correct name for Sn 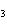 (PO 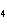 ) 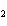 ? a. | tritin diphosphate | c. | tin(III) phosphate | b. | tin(II) phosphate | d. | tin(IV)
phosphate |
|
|
|
The word Nomenclature comes from Nomenclator, who is a person who calls out
names. "Nomen" means names and "clator" is a person who calls out. When
students get their diploma, the nomenclator is the person who calls out the students'
names. In chemistry, "nomenclature" is learning to call out chemical
names. Understanding chemistry nomenclature is like learning any language. It takes
practice and study, but the reward is that it gives you the ability to talk to people you
couldn't talk to before.
|
|
|
33.
|
What does the term nomenclature mean?
a. | learning to call out chemical names | c. | to mold from
clay | b. | writing formulas | d. | to practice and study |
|
|
|
|
|
|
34.
|
What is the stock system name for the ionic compound stannous fluoride?
a. | can’t be names using the stock system | c. | tin (II)
fluoride | b. | tin (IV) fluoride | d. | stannic fluoride |
|
|
|
Use the following information (and the table
above) for the next two questions.
Cuprous oxide is commonly used as a pigment, a
fungicide, and an antifouling agent for marine paints. Rectifier diodes based on this material have
been used industrially as early as 1924, long before silicon became the standard.
|
|
|
35.
|
What is the charge of the copper in cuprous oxide?
|
|
|
36.
|
What is the correct stock system name for cuprous oxide.
a. | Can’t be determined | c. | copper (II)
oxide | b. | copper (I) oxide | d. | copper oxide |
|