Chemistry Ch. 1 & 2 Prep Test
Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
1.
|
Chemistry may be least useful in studying
a. | matter. | c. | falling bodies. | b. | synthetic fibers. | d. | medicine. |
|
|
|
2.
|
Which state of matter takes both the shape and volume of its container?
a. | solid | c. | gas | b. | liquid | d. | both b and c |
|
|
|
3.
|
Which action changes the identity of the substance referenced?
a. | melting gold | b. | running an electric current through
copper | c. | corroding iron | d. | breaking an ice
cube |
|
|
|
4.
|
A vapor is which state of matter?
a. | solid | c. | gas | b. | liquid | d. | all of the
above |
|
|
|
5.
|
Which of the following is a chemical property?
a. | color | c. | freezing point | b. | hardness | d. | ability to react with
oxygen |
|
|
|
6.
|
Which state of matter expands when heated and is easy to compress?
a. | gas | c. | solid | b. | liquid | d. | all of the
above |
|
|
|
7.
|
Which of the following is a heterogeneous mixture?
a. | air | c. | steel | b. | salt water | d. | soil |
|
|
|
8.
|
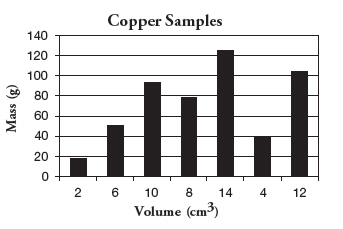 Which of the following is the independent
variable in this graph? a. | Mass (g) | c. | Copper Samples | b. | Volume (cm3) | d. | None of these |
|
|
|
9.
|
What must occur for a change to be a chemical reaction?
a. | There must be a change in chemical properties. | b. | There must be a
change in physical properties. | c. | The change must involve a change in
mass. | d. | The change must involve a change in volume. |
|
|
|
10.
|
Which of the following is a homogeneous mixture?
a. | blood | c. | oil and vinegar | b. | milk | d. | air |
|
|
|
11.
|
Which of the following processes does NOT involve a change in chemical
properties?
a. | rusting | c. | boiling | b. | fermenting | d. | burning |
|
|
|
12.
|
Chemistry is defined as the study of the composition and structure of materials
and
a. | the categories of matter. | c. | the electrical currents in
matter. | b. | the changes in matter. | d. | molecules in living things. |
|
|
|
13.
|
What must be done to be certain that a chemical change has taken place?
a. | Check for the production of bubbles before and after the change. | b. | Demonstrate that a
release of energy occurred after the change. | c. | Check the composition of the sample before and
after the change. | d. | Demonstrate that energy was absorbed by the
reactants after the change. |
|
|
|
14.
|
What is one difference between a mixture and a compound?
a. | A compound consists of more than one phase. | b. | A compound can only
be separated into its components by chemical means. | c. | A mixture can only be separated into its
components by chemical means. | d. | A mixture must be uniform in
composition. |
|
|
|
15.
|
Chemistry is the study of all of the following EXCEPT
a. | matter. | c. | energy associated with changes in matter. | b. | changes in
matter. | d. | projectile
motion. |
|
|
|
16.
|
All of the following are physical properties of a substance in the liquid state
EXCEPT ____.
a. | indefinite volume | c. | not easily compressed | b. | definite
mass | d. | indefinite
shape |
|
|
|
|
|
|
17.
|
The homogeneous mixture in the illustration above is in container
|
|
|
18.
|
Under ordinary conditions of temperature and pressure, the particles in a gas
are
a. | closely packed. | c. | held in fixed positions. | b. | very far from each
other. | d. | able to slide past
each other. |
|
|
|
19.
|
Which of the following is true about homogeneous mixtures?
a. | They are known as solutions. | b. | They consist of two or more
phases. | c. | They have compositions that never vary. | d. | They are always
liquids. |
|
|
|
20.
|
Which of the following is only a physical change?
a. | corrosion | c. | evaporation | b. | explosion | d. | rotting of food |
|
|
|
21.
|
Which of the following would a chemist be most likely to study?
a. | a leaf floating on water | c. | a leaf being blown by the
wind | b. | a leaf changing color in autumn | d. | a leaf being eaten by
insects |
|
|
|
22.
|
Which of the following does NOT involve just a physical change?
a. | mixing | c. | grinding | b. | melting | d. | decomposing |
|
|
|
23.
|
Chemistry may be most useful in studying
a. | the movement of asteroids. | c. | eating habits of
ducks. | b. | why materials corrode. | d. | streamlining of race cars. |
|
|
|
|
|
|
24.
|
Which of the following is NOT a controlled variable?
a. | Initial Temperature | c. | Number of Alka-seltzer tablets | b. | Volume | d. | Room
pressure |
|
|
|
25.
|
Which of the following variables is the DEPENDENT variable?
a. | Volume of vinegar | c. | Final Temperature | b. | Number of Alka-seltzer
tablets | d. | Room
Pressure |
|
|
|
26.
|
What is the change in temperature (DT) of the
solution in trial 3?
a. | 23.5 oC | c. | 2.9 oC | b. | 20.3 oC | d. | - 3.1
oC |
|
|
|
27.
|
The state of matter in which particles are rigidly held in fixed positions is
the
a. | gaseous state. | c. | vaporous state. | b. | liquid state. | d. | solid state. |
|
|
|
28.
|
An example of a homogeneous mixture is ____.
a. | distilled water | c. | noodle soup | b. | stainless steel | d. | oxygen |
|
|
|
29.
|
Which of the following is true about compounds?
a. | They can be physically separated into their component elements. | b. | They have
compositions that vary. | c. | They are substances. | d. | They have properties
similar to those of their component elements. |
|
|
|
30.
|
Which of the following indicates that a chemical change has happened during
cooking?
a. | The food changes color. | b. | Bubbles form in boiling
water. | c. | Butter melts. | d. | Energy is transferred from the stove to a
pan. |
|
|
|
31.
|
The study of matter and changes in matter best describes the science of
a. | biology. | c. | microbiology. | b. | physics. | d. | chemistry. |
|
|
|
32.
|
Which of the following is not a mixture.
a. | cookie dough. | c. | distilled water | b. | blood | d. | air |
|
|
|
33.
|
What happens to matter during a chemical reaction?
a. | Matter is neither destroyed or created. | b. | Some matter is
destroyed. | c. | Some matter is created. | d. | Some matter is destroyed and some is
created. |
|
|
|
34.
|
Which state of matter is characterized by having an indefinite shape, but a
definite volume?
a. | gas | c. | solid | b. | liquid | d. | none of the
above |
|
|
|
35.
|
Which state of matter is characterized by having a definite shape and a definite
volume?
a. | gas | c. | solid | b. | liquid | d. | all of the
above |
|
Matching
|
|
|
Match each item with the correct statement below. a. | mixture | d. | reactant | b. | product | e. | heterogeneous mixture | c. | chemical
change | f. | vapor |
|
|
|
36.
|
gaseous state of substance that is a liquid or solid at room
temperature
|
|
|
37.
|
not uniform in composition
|
|
|
38.
|
composition differs between reactants and products
|
|
|
39.
|
a physical blend of two or more components
|
|
|
40.
|
a substance formed in a chemical reaction
|
|
|
41.
|
starting substance in a chemical reaction
|