Multiple Choice
Identify the letter of the choice that best
completes the statement or answers the question.
|
|
|
|
|
|
1.
|
Which of the illustrations above represents a fission reaction?
|
|
|
2.
|
Which of the following is a fusion reaction?
a. | uranium-235 absorbing a neutron and splitting into xenon-140, strontium-94, and two
neutrons | b. | hydrochloric acid combining with sodium hydroxide to form NaCl and
water | c. | carbon-14 decaying into nitrogen-14 and a beta particle | d. | curium-246 combining
with carbon-12 to form nobelium-254 and four neutrons |
|
|
|
3.
|
The energy released in a nuclear reaction comes from
a. | electrons. | c. | positrons. | b. | bonds. | d. | the binding energy of the
nucleus. |
|
|
|
4.
|
Particles or electromagnetic radiation emitted from the nucleus during
radioactive decay
a. | is harmless nuclear fallout. | c. | is
transmutation. | b. | is nuclear radiation. | d. | are daughter nuclides. |
|
|
|
5.
|
Which of the following processes always decreases the number of protons by an
even number?
a. | fusion | c. | alpha decay | b. | beta decay | d. | fission |
|
|
|
6.
|
Which of the following lists ranks nuclear radiation from most massive to least
massive?
a. | alpha, beta, and gamma | c. | gamma, alpha, and beta | b. | beta, gamma, and
alpha | d. | gamma, beta, and
alpha |
|
|
|
7.
|
What is the half-life of an isotope if 125 g of a 500 g sample of the isotope
remains after 3.0 years?
a. | 1.5 years | c. | 3.5 years | b. | 2.5 years | d. | 4.5 years |
|
|
|
8.
|
Which series consists of radioactive nuclides produced by successive radioactive
decay until a stable nuclide is reached?
a. | parent series | c. | nuclide series | b. | half-life series | d. | decay series |
|
|
|
9.
|
Which of the following has the weakest penetration abilty?
a. | alpha particles | c. | gamma rays | b. | beta particles | d. | All have the same penetrating
ability. |
|
|
|
10.
|
Which of the following has the greatest penetrating ability?
a. | alpha particles | c. | gamma rays | b. | beta particles | d. | All have the same penetrating
ability. |
|
|
|
11.
|
Which of the following is the purpose of a Geiger counter?
a. | illustrate a decay series | c. | record stokres on the golf
course | b. | record half lives | d. | detect radiation |
|
|
|
12.
|
Which of the following is a fission reaction?
a. | hydrogen-2 and hydrogen-3 combining to form a helium-4 atom and a
neutron | b. | carbon-12 and hydrogen-1 combining to form a nitrogen-13 atom | c. | uranium-235
absorbing a neutron and breaking into barium-141, krypton-92, and three neutrons | d. | a glucose molecule
being metabolized with oxygen to form carbon dioxide and water |
|
|
|
13.
|
An unstable nucleus ____.
a. | increases its nuclear mass by fission | c. | emits energy when it
decays | b. | increases its half-life | d. | expels all of its protons |
|
|
|
14.
|
What particle is emitted in alpha radiation?
a. | electron | c. | helium nucleus | b. | photon | d. | hydrogen
nucleus |
|
|
|
15.
|
What is the change in the atomic number when an atom emits an alpha
particle?
a. | decreases by 2 | c. | increases by 1 | b. | decreases by 1 | d. | increases by 2 |
|
|
|
16.
|
What is the change in atomic number when an atom emits a beta particle?
a. | decreases by 2 | c. | increases by 2 | b. | decreases by 1 | d. | increases by 1 |
|
|
|
17.
|
Which symbol is used for an alpha particle?
|
|
|
18.
|
What symbol is used for beta radiation?
|
|
|
19.
|
What particle is needed to complete this nuclear reaction? 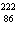 Rn
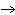 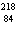 Po + _____
|
|
|
20.
|
When radium-226 (atomic number 88) decays by emitting an alpha particle, it
becomes ____.
a. | polonium-222 | c. | radium-222 | b. | polonium-224 | d. | radon-222 |
|
|
|
21.
|
What particle does argon-39 (atomic number 18) emit when it decays to
potassium-39 (atomic number 19)?
a. | neutron | c. | proton | b. | electron | d. | alpha particle |
|
|
|
22.
|
What particle is needed to complete the following nuclear equation?  Mn 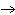 ____ + 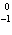 e
|
|
|
23.
|
What particle is needed to complete the following equation? 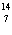 N +
____ 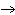 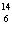 C + 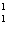 H
|
|
|
24.
|
To what element does polonium-208 (atomic number 84) decay when it emits an
alpha particle?
|
|
|
25.
|
Which of the following is the parent nuclide? 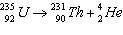
|
|
|
26.
|
Which of the following is the daughter nucleus? 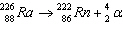
|
|
|
27.
|
The half-life of radon-222 is about four days. What percent of a sample would
you expect to find after 12 days?
|
|
|
28.
|
If the half-life of sodium-24 is 15 hours, how much remains from a 10.0-g sample
after 60 hours?
a. | 0.625g | c. | 0.313g | b. | 2.50g | d. | 1.25g |
|
|
|
|
|
|
29.
|
Consider the diagram above. Which letter best represents the penetrating ability
of beta particles?
a. | A | c. | C | b. | B | d. | none are
correct |
|
|
|
30.
|
Protons and neutrons have substructures and consist of particles called
a. | quarks. | c. | leptons | b. | marks | d. | brotrons |
|
Matching
|
|
|
Match each item with the correct statement below. a. | positron | d. | transuranium element | b. | alpha particle | e. | gamma radiation | c. | beta
particle |
|
|
|
31.
|
element with atomic number greater than 92
|
|
|
32.
|
emitted helium nucleus
|
|
|
33.
|
negatively charged radioactive particle.
|
|
|
34.
|
high-energy photons emitted by a radioisotope
|
|
|
35.
|
particle of charge +1 and mass equal to that of an electron
|
|
|
Match each item with the correct statement below. a. | fission | c. | Geiger counter | b. | fusion | d. | radioisotope |
|
|
|
36.
|
element with unstable nucleus
|
|
|
37.
|
combination of two nuclei to form a nucleus of greater mass
|
|
|
38.
|
radiation detector that makes use of a gas-filled metal tube
|
|
|
39.
|
splitting of nucleus into smaller fragments
|