Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
1.
|
A chemical reaction has NOT occurred if the products have
a. | the same mass as the reactants. | b. | less total bond energy than the
reactants. | c. | more total bond energy than the reactants. | d. | the same chemical
properties as the reactants. |
|
|
|
2.
|
Which observation does NOT indicate that a chemical reaction has
occurred?
a. | formation of a precipitate | c. | evolution of heat and
light | b. | production of a gas | d. | change in total mass of substances |
|
|
|
3.
|
A solid produced by a chemical reaction in solution that separates from the
solution is called
a. | a precipitate. | c. | a molecule. | b. | a reactant. | d. | the mass of the
product. |
|
|
|
4.
|
After the correct formula for a reactant in an equation has been written,
the
a. | subscripts are adjusted to balance the equation. | b. | formula should not
be changed. | c. | same formula must appear as the product. | d. | symbols in the
formula must not appear on the product side of the equation. |
|
|
|
5.
|
In writing an equation that produces hydrogen gas, the correct representation of
hydrogen gas is
|
|
|
6.
|
What is the small whole number that appears in front of a formula in a chemical
equation?
a. | a subscript | c. | a ratio | b. | a superscript | d. | a coefficient |
|
|
|
7.
|
To balance a chemical equation, it may be necessary to adjust the
a. | coefficients. | c. | formulas of the products. | b. | subscripts. | d. | number of products. |
|
|
|
8.
|
A chemical equation is balanced when the
a. | coefficients of the reactants equal the coefficients of the
products. | b. | same number of each kind of atom appears in the reactants and in the
products. | c. | products and reactants are the same chemicals. | d. | subscripts of the
reactants equal the subscripts of the products. |
|
|
|
9.
|
How would oxygen be represented in the formula equation for the reaction of
methane and oxygen to yield carbon dioxide and water?
|
|
|
10.
|
Which of the following is a formula equation for the formation of carbon dioxide
from carbon and oxygen?
a. | Carbon plus oxygen yields carbon dioxide. | b. | C + O2
® CO2 | c. | CO2 ® C + O2 | d. | 2C + O ®
CO2 |
|
|
|
11.
|
In an equation, the symbol for a substance in water solution is followed
by
a. | (1). | c. | (aq). | b. | (g). | d. | (s). |
|
|
|
12.
|
When the equation Fe3O4 + Al ® Al2O3 + Fe is correctly balanced, what is the
coefficient of Fe?
|
|
|
13.
|
Which coefficients correctly balance the formula equation
NH4NO2(s)® N2(g) +
H2O(l)?
a. | 1, 2, 2 | c. | 2, 1, 1 | b. | 1, 1, 2 | d. | 2, 2, 2 |
|
|
|
14.
|
Which coefficients correctly balance the formula equation CaO + H2O
® Ca(OH)2?
a. | 2, 1, 2 | c. | 1, 2, 1 | b. | 1, 2, 3 | d. | 1, 1, 1 |
|
|
|
15.
|
After the first steps in writing an equation, the equation is balanced by
a. | adjusting subscripts to the formula(s). | b. | adjusting
coefficients to the smallest whole-number ratio. | c. | changing the products
formed. | d. | making the number of reactants equal to the number of
products. |
|
|
|
16.
|
The complete balanced equation for the reaction between zinc hydroxide and
acetic acid is
a. | ZnOH + CH3COOH ® ZnCH3COO +
H2O. | b. | Zn(OH)2 + CH3COOH ® Zn +
2CO2 +3H2O. | c. | Zn(OH)2 + 2CH3COOH ® Zn(CH3COO)2 + 2H2O. | d. | Zn(OH)2 +
2CH3COOH ® Zn(CH3COO)2 +
H2 + O2. |
|
|
|
17.
|
What is the balanced equation for the combustion of sulfur?
a. | S(s) + O2(g) ®
SO(g) | c. | 2S(s) + 3O2(g) ®
SO3(s) | b. | S(s) + O2(g) ® SO2(g) | d. | S(s) + 2O2(g) ®
SO42–(aq) |
|
|
|
18.
|
Chemical reactions ____.
a. | occur only in living organisms | c. | only occur outside living
organisms | b. | create and destroy atoms | d. | produce new substances |
|
|
|
19.
|
Chemical equations ____.
a. | describe chemical reactions | b. | show how to write chemical
formulas | c. | give directions for naming chemical compounds | d. | describe only
biological changes |
|
|
|
20.
|
Symbols used in equations, together with the explanations of the symbols, are
shown below. Which set is correct?
a. | (g), grams | c. | (aq), dissolved in water | b. | (l),
liters | d. | (s), solid
product |
|
|
|
21.
|
In the chemical equation H 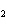 O 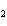 ( aq) ® H 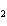 O( l) 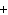 O 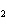 ( g), the 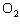 is a ____. a. | catalyst | c. | product | b. | solid | d. | reactant |
|
|
|
22.
|
Which of the following is the correct skeleton equation for the reaction that
takes place when solid phosphorus combines with oxygen gas to form diphosphorus pentoxide?
|
|
|
23.
|
If you rewrite the following word equation as a balanced chemical equation, what
will the coefficient and symbol for fluorine be? nitrogen trifluoride 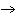 nitrogen 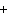 fluorine
|
|
|
24.
|
What are the coefficients that will balance the skeleton equation
below? AlCl 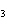 + NaOH 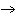 Al(OH) 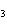 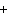 NaCl a. | 1, 3, 1, 3 | c. | 1, 1, 1, 3 | b. | 3, 1, 3, 1 | d. | 1, 3, 3, 1 |
|
|
|
25.
|
What are the coefficients that will balance the skeleton equation below?
N 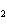 + H 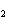 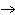 NH 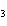 a. | 1, 1, 2 | c. | 3, 1, 2 | b. | 1, 3, 3 | d. | 1, 3, 2 |
|
|
|
26.
|
When the equation Fe 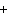 Cl 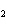 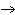 FeCl 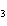 is
balanced, what is the coefficient for Cl 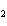 ?
|
|
|
27.
|
When the following equation is balanced, what is the coefficient for HCl?
Mg( s) 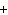 HCl( aq) 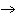 MgCl 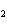 ( aq) 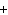 H 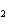 ( g)
|
|
|
28.
|
Which of the following statements is NOT true about what happens in all chemical
reactions?
a. | The ways in which atoms are joined together are changed. | b. | New atoms are formed
as products. | c. | The starting substances are called reactants. | d. | The bonds of the
reactants are broken and new bonds of the products are formed. |
|
|
|
29.
|
When the equation KClO 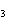 ( s)  KCl( s) + O 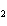 ( g) is balanced, the coefficient of KClO 3 is ____.
|
|
|
30.
|
In every balanced chemical equation, each side of the equation has the same
number of ____.
a. | atoms of each element | c. | moles | b. | molecules | d. | coefficients |
|
|
|
31.
|
What are the missing coefficients for the skeleton equation
below? Cr( s) 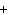 Fe(NO 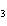 ) 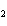 ( aq) 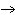 Fe( s) 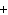 Cr(NO 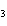 ) 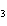 ( aq) a. | 4, 6, 6, 2 | c. | 2, 3, 3, 2 | b. | 2, 3, 2, 3 | d. | 1, 3, 3, 1 |
|
|
|
32.
|
What are the missing coefficients for the skeleton equation below?
Al 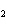 (SO 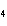 ) 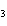 ( aq) 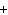
KOH( aq) ® Al(OH) 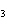 ( aq) 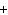 K 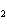 SO 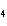 ( aq) a. | 1, 3, 2, 3 | c. | 4, 6, 2, 3 | b. | 2, 12, 4, 6 | d. | 1, 6, 2, 3 |
|
|
|
33.
|
When potassium hydroxide and barium chloride react, potassium chloride and
barium hydroxide are formed. The balanced equation for this reaction is ____.
|
|
|
34.
|
The product of a combination reaction is Ba(OH) 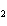 . If one
of the reactants is H 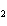 O, what is the other reactant? a. | Ba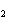 O O | c. | BaH | b. | BaO | d. | BaO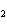 |
|
|
|
35.
|
For the formula equation 2Mg + O2 ®
2MgO, the word equation would begin
a. | Manganese plus oxygen . . . | c. | Magnesium plus oxygen . .
. | b. | Molybdenum plus oxygen . . . | d. | Heat plus oxygen . .
. |
|
Matching
|
|
|
Match each item with the correct statement below. a. | product | d. | balanced equation | b. | reactant | e. | skeleton equation | c. | chemical
equation |
|
|
|
36.
|
a chemical equation that does not indicate relative amounts of reactants and
products
|
|
|
37.
|
a new substance formed in a chemical reaction
|
|
|
38.
|
a starting substance in a chemical reaction
|
|
|
39.
|
a concise representation of a chemical reaction
|
|
|
40.
|
an equation in which each side has the same number of atoms of each
element
|