Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
1.
|
If you start with 10.0 moles of C3H8 (propane) and 10.0
moles of O2, what is the limiting
reactant.
C3H8(g) + 5O2(g) ®
3CO2(g) + 4H2O(g)
a. | oxygen | c. | carbon dioxide | b. | propane | d. | water |
|
|
|
2.
|
For the reaction 2Na + Cl2 ® 2NaCl,
how many grams of sodium chloride can be produced from 142. g of chlorine?
a. | 117 g | c. | 400 g | b. | 234 g | d. | 825 g |
|
|
|
3.
|
How many moles of Al 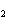 O 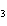 are produced when 0.60 mol of Fe is made in the following
reaction.
2Al( s) + 3FeO( s) 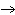 3Fe( s) + Al 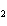 O 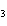 ( s) a. | 0.20 mol | c. | 0.60 mol | b. | 0.40 mol | d. | 0.90 mol |
|
|
|
4.
|
In the reaction C + 2H2 ®
CH4, what is the mole ratio of hydrogen to methane?
|
|
|
5.
|
In the reaction N2 + 3H2 ®
2NH3, what is the mole ratio of hydrogen to NH3?
|
|
|
6.
|
For the reaction CH4 + 2O2 ® 2H2O + CO2, calculate the percent yield of
carbon dioxide if 1600. g of methane react with excess oxygen to produce 2200. g of carbon
dioxide.
a. | 50.00% | c. | 100.0% | b. | 80.00% | d. | 90.00% |
|
|
|
7.
|
For the reaction Cl2 + 2KBr ® 2KCl +
Br2, calculate the percent yield if 213 g of chlorine reacts with
excess potassium bromide to produce 432 g of bromine.
a. | 70.0% | c. | 90.0% | b. | 100.% | d. | 80.0% |
|
|
|
8.
|
In the reaction Zn + H2SO4 ® ZnSO4 + H2, what is the mole ratio of zinc to
sulfuric acid?
|
|
|
9.
|
How many moles of aluminum are needed to react completely with 1.5 mol of FeO?
2Al( s) + 3FeO( s) ® 3Fe( s) + Al 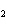 O 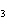 ( s) a. | 1.5 mol | c. | 3.0 mol | b. | 1.0 mol | d. | 0.5 mol |
|
|
|
10.
|
Iron(III) oxide is formed when iron combines with oxygen in the air. How many
grams of Fe 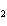 O 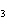 are
formed when 27.9 g of Fe reacts completely with oxygen? 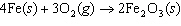 a. | 12.0 g | c. | 30.0 g | b. | 40.0 g | d. | 95.0 g |
|
|
|
11.
|
For the reaction 2Zn + O2 ® 2ZnO, how
many grams of zinc oxide can be produced from 196 g of zinc?
a. | 100. g | c. | 122 g | b. | 244 g | d. | 200. g |
|
|
|
12.
|
For the reaction SO3 + H2O ® H2SO4, how many grams of sulfur trioxide are
required to produce 4.00 mol of sulfuric acid?
a. | 80.0 g | c. | 240. g | b. | 160. g | d. | 320. g |
|
|
|
13.
|
In the reaction 2CO( g) + O 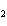 ( g) ® 2CO 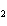 ( g), what is the ratio of moles
of oxygen used to moles of CO 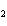 produced?
|
|
|
14.
|
The Haber process for producing ammonia (NH3) commercially is
represented by the equation :
N2(g) + 3H2(g) ® 2NH3(g).
To completely convert 9.0 mol hydrogen gas
to ammonia gas, how many moles of nitrogen gas are required?
a. | 1.0 mol | c. | 3.0 mol | b. | 2.0 mol | d. | 6.0 mol |
|
|
|
15.
|
For the reaction 2KClO3 ® 2KCl +
3O2, how many moles of potassium chlorate are required to produce 96 g of oxygen?
a. | 2.0 mol | c. | 4.0 mol | b. | 4.5 mol | d. | 5.0 mol |
|
|
|
16.
|
For the reaction 2Na + 2H2O ® 2NaOH +
H2, how many grams of sodium hydroxide are produced from 3.0 moles of water?
a. | 40. g | c. | 120 g | b. | 80. g | d. | 240 g |
|
|
|
17.
|
In the chemical reaction wA + xB ® yC + zD, a
comparison of the number of moles of A to the number of moles of C would be a(n)
a. | mass ratio. | c. | electron ratio. | b. | mole ratio. | d. | energy
proportion. |
|
|
|
18.
|
The coefficients in a chemical equation represent the
a. | masses, in grams, of all reactants and products. | b. | relative numbers of
moles of reactants and products. | c. | number of atoms in each compound in a
reaction. | d. | number of valence electrons involved in the reaction. |
|
|
|
19.
|
For the reaction Pb(NO3)2 + 2KI ® PbI2 + 2KNO3, how many moles of lead iodide are
produced from 332. g of potassium iodide?
a. | 1.00 mol | c. | 3.00 mol | b. | 1.50 mol | d. | 10.0 mol |
|
|
|
20.
|
What is the maximum possible amount of product obtained in a chemical
reaction?
a. | theoretical yield | c. | mole ratio | b. | percent yield | d. | actual yield |
|
|
|
21.
|
For the reaction 2HNO3 + Mg(OH)2 ® Mg(NO3)2 + 2H2O, how many grams of
magnesium nitrate are produced from 4.00 mol of nitric acid?
a. | 148 g | c. | 296 g | b. | 445 g | d. | 592 g |
|
Matching
|
|
|
Match each item with the correct statement below. a. | actual/experimental yield | e. | limiting
reagent | b. | percent yield | f. | mass | c. | theoretical yield | g. | number of molecules | d. | excess
reagent | h. | volume |
|
|
|
22.
|
the reactant that is not completely used up in a reaction
|
|
|
23.
|
the amount of product formed when a reaction is carried out in the
laboratory
|
|
|
24.
|
the reactant that determines the amount of product that can be formed in a
reaction
|
|
|
25.
|
This is conserved in every ordinary chemical reaction.
|
|
|
26.
|
the maximum amount of product that could be formed from given amounts of
reactants
|
|
|
27.
|
the ratio of the actual yield to the theoretical yield
|