Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
A conjugate base is the species that
a. | remains after a base has given up a proton. | b. | is formed by the
addition of a proton to a base. | c. | is formed by the addition of a proton to an
acid. | d. | remains after an acid has given up a proton. |
|
|
|
2.
|
A substance that ionizes nearly completely in aqueous solutions and
produces H+ is a
a. | weak base. | c. | weak acid. | b. | strong base. | d. | strong acid. |
|
|
|
3.
|
In the reaction H 3PO 4 + H 2O 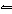
H 3O + + H 2PO 4–, the molecule
H 2O acts as a(n) a. | acid. | c. | spectator species. | b. | base. | d. | salt. |
|
|
|
4.
|
The substances produced when KOH(aq) neutralizes HCl(aq)
are
a. | HClO(aq) and KH(aq). | c. | H2O(l) and
KCl(aq). | b. | KH2O+(aq) and
Cl–(aq). | d. | H3O+(aq) and
KCl(aq). |
|
|
|
5.
|
The pH of a solution is 12. What is its
[OH-]?
a. | 1 x 10–12 M | c. | 0.01 M | b. | 1 x
10–7 M | d. | 12
M |
|
|
|
6.
|
A species that is formed when a base gains a proton is a
a. | conjugate base. | c. | strong base. | b. | conjugate acid. | d. | strong acid. |
|
|
|
7.
|
What is the pH of a 0.001 M KOH solution?
|
|
|
8.
|
In the reaction HClO 3 + NH 3 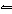
NH 4+ + ClO 3–, the conjugate base of
HClO 3 is a. | ClO3–. | c. | NH4+. | b. | NH3. | d. | not shown. |
|
|
|
9.
|
Which expression represents the pH of a solution?
a. | log[H+] | c. | log[OH–] | b. | –
log[H+] | d. | – log[OH–] |
|
|
|
10.
|
Acids make blue litmus paper turn
a. | red. | c. | blue. | b. | yellow. | d. | black. |
|
|
|
11.
|
Which of the following is a diprotic acid?
a. | H2SO4 | c. | HCl | b. | CH3COOH | d. | H3PO4 |
|
|
|
12.
|
The pH scale in general use ranges from
a. | 0 to 1. | c. | 0 to 7. | b. | –1 to 1. | d. | 0 to 14. |
|
|
|
13.
|
In the equation HCl(g) + H2O(l) ® H3O+(aq) + Cl–(aq),
which species is a Brønsted-Lowry acid?
a. | HCl | c. | Cl– | b. | Na2O | d. | none of the
above |
|
|
|
14.
|
A species that can react as either an acid or a base is a(n)
a. | Lewis acid. | c. | oxyacid. | b. | amphoteric substance. | d. | organic
substance. |
|
|
|
|
|
|
15.
|
All acid base reactions have two conjugate acid base pairs. What is one
conjugate acid base pair in the above reaction?
a. | H2O/H3O1+ | c. | CO32-/H3O1+ | b. | H2O/HCO31- | d. | H2O/OH1- |
|
|
|
16.
|
If [H+] of a solution is greater than
[OH–], the solution
a. | is always acidic. | c. | is always neutral. | b. | is always basic. | d. | might be acidic, basic, or
neutral. |
|
|
|
17.
|
Acids taste
a. | sweet. | c. | bitter. | b. | sour. | d. | salty. |
|
|
|
18.
|
If [H+] = 1.00 x 10–5 M, what is the pH of
the solution?
|
|
|
19.
|
The traditional (1st) definition of acids is based on the observations of
a. | Brønsted and Lowry. | c. | Arrhenius. | b. | Lewis. | d. | Faraday. |
|
|
|
20.
|
Acids react with
a. | bases to produce salts and water. | c. | water to produce bases and
salts. | b. | salts to produce bases and water. | d. | neither bases, salts, nor
water. |
|
|
|
21.
|
Which of the following is NOT a strong acid?
a. | HNO3 | c. | H2SO4 | b. | CH3COOH | d. | HCl |
|
|
|
22.
|
Bases feel
a. | rough. | c. | slippery. | b. | moist. | d. | dry. |
|
|
|
23.
|
In the reaction HClO 3 + NH 3 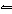
NH 4+ + ClO 3–, the conjugate base of NH 3
is a. | HClO3. | c. | NH4+. | b. | ClO3–. | d. | not shown. |
|
|
|
24.
|
In the reaction HSO 4– + H 2O 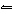 H 3O + + SO 42–, the ion
HSO 4– acts as a(n) a. | acid. | c. | spectator species. | b. | base. | d. | salt. |
|
|
|
Solution | [H+]
3 | [OH–] | pH | pOH | A | 1×10–3 M | 1×10–11 M | 3.0 | 11.0 | B | 1×10–9 M | 1×10–5 M | 9.0 | 5.0 | C | 5.2×10–3 M | 1.9×10–12 M | 2.28 | 11.72 | D | | | | 3.68 | E | | | 9.28 | | F | | 3.02×10–3 M | | | | | | | |
|
|
|
25.
|
What is the [H+] from Solution D above?
a. | 2.1 x 10-4 M | c. | 4.8 x 10-11
M | b. | 3.1 x 10-6 M | d. | 1.9 x 10-12 M |
|
|
|
26.
|
What is the hydrogen ion concentration of a solution whose pH is 4.00?
a. | 1.0 x 10–10 M | c. | 6.0 x 10–4
M | b. | 5.0 x 10–6 M | d. | 1.0 x 10–4
M |
|
|
|
27.
|
Pure water can partially break down into charged particles in a process
called
a. | hydration. | c. | self-ionization. | b. | hydrolysis. | d. | dissociation. |
|
|
|
28.
|
A Brønsted-Lowry acid is
a. | an electron-pair acceptor. | c. | a proton
acceptor. | b. | an electron-pair donor. | d. | a proton donor. |
|
|
|
29.
|
In the reaction HF + H 2O  H 3O + +
F –, a conjugate acid-base pair is a. | HF and H2O. | c. | H3O+ and H2O. | b. | F– and
H3O+. | d. | HF and H3O+. |
|
|
|
30.
|
The pH of a basic solution is
a. | less than 0. | c. | greater than 7. | b. | less than 7. | d. | greater than
14. |
|