Period 2 Ch. 4 Prep Test
Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
1.
|
Compared with acids that have the suffix -ic, acids that have the suffix -ous
contain
a. | more hydrogen. | c. | less oxygen. | b. | more oxygen. | d. | the same amount of
oxygen. |
|
|
|
2.
|
A chemical formula includes the symbols of the elements in the compound and
subscripts that indicate
a. | the number of electrons in each element. | b. | how many atoms or
ions of each type are combined in the simplest unit. | c. | the formula mass. | d. | the charges on the
elements or ions. |
|
|
|
3.
|
Changing a subscript in a correctly written chemical formula
a. | changes the number of protons represented by the formula. | b. | changes the charges
on the other ions in the compound. | c. | changes the formula so that it no longer
represents that compound. | d. | has no effect on the
formula. |
|
|
|
4.
|
What is the formula for the compound formed by lead(II) ions and chromate
ions?
a. | PbCrO4 | c. | Pb2(CrO4)3 | b. | Pb2CrO4 | d. | Pb(CrO4)2 |
|
|
|
5.
|
What is the formula for tin(IV) chromate?
a. | Sn(CrO4)4 | c. | Sn2(CrO4)4 | b. | Sn2(CrO4)2 | d. | Sn(CrO4)2 |
|
|
|
6.
|
Name the compound CuCO3.
a. | copper(I) carbonate | c. | copper(IV) carbonate | b. | copper(III) carbonate | d. | copper(II)
carbonate |
|
|
|
7.
|
What type of ions have names ending in -ide?
a. | only cations | c. | only metal ions | b. | only anions | d. | only gaseous
ions |
|
|
|
8.
|
When Group 2A elements form ions, they ____.
a. | lose two protons | c. | lose two electrons | b. | gain two protons | d. | gain two
electrons |
|
|
|
9.
|
What is the correct name for the N 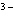
ion? a. | nitrate ion | c. | nitride ion | b. | nitrogen ion | d. | nitrite ion |
|
|
|
10.
|
When naming a transition metal ion that can have more than one common ionic
charge, the numerical value of the charge is indicated by a ____.
a. | prefix | c. | Roman numeral following the name | b. | suffix | d. | superscript after the name |
|
|
|
11.
|
Aluminum is a group 3 (or 13) metal. Which ion does Al typically form?
|
|
|
12.
|
Which of the following correctly provides the names and formulas of polyatomic
ions?
|
|
|
13.
|
An -ate or -ite at the end of a compound name usually indicates
that the compound contains ____.
a. | fewer electrons than protons | c. | only 1 element | b. | neutral
molecules | d. | a polyatomic
anion |
|
|
|
14.
|
Which of the following compounds contains the Mn 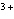 ion?
|
|
|
15.
|
Which of the following is true about the composition of ionic compounds?
a. | They are composed of anions and cations. | b. | They are composed of
anions only. | c. | They are composed of cations only. | d. | They are formed from two or more nonmetallic
elements. |
|
|
|
16.
|
Which of the following formulas represents an ionic compound?
|
|
|
17.
|
Which element, when combined with fluorine, would most likely form an ionic
compound?
a. | lithium | c. | phosphorus | b. | carbon | d. | chlorine |
|
|
|
18.
|
Which of the following correctly represents an ion pair and the ionic compound
the ions form?
|
|
|
19.
|
In which of the following is the name and formula given correctly?
a. | sodium oxide, NaO | c. | cobalt (I) chloride, CoCl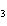 | b. | barium nitride, BaN | d. | Tin (IV) fluoride, SnF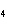 |
|
|
|
20.
|
Which of the following compounds contains the lead(II) ion?
|
|
|
21.
|
What is the correct formula for potassium sulfite?
|
|
|
22.
|
Which set of chemical name and chemical formula for the same compound is
correct?
a. | ammonium sulfite, (NH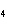 ) )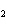 S S | c. | lithium carbonate, LiCO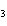 | b. | iron(III) phosphate,
FePO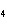 | d. | magnesium dichromate, MgCrO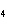 |
|
|
|
23.
|
What is the ending for the names of all binary compounds, both ionic and
molecular?
|
|
|
24.
|
Which of the following correctly shows a prefix used in naming binary molecular
compounds with its corresponding number?
a. | deca-, 7 | c. | hexa-, 8 | b. | nona-, 9 | d. | octa-, 4 |
|
|
|
25.
|
Which of the following formulas represents a molecular compound?
a. | ZnO | c. | SO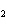 | b. | Xe | d. | BeF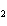 |
|
|
|
26.
|
Which of the following shows both the correct formula and correct name of an
acid?
a. | HClO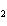 , chloric acid , chloric acid | c. | H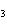 PO PO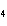 , phosphoric acid , phosphoric acid | b. | HNO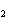 , hydronitrous acid , hydronitrous acid | d. | HI, iodic acid |
|
|
|
27.
|
What is the name of H 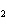 SO 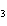 ? a. | hyposulfuric acid | c. | sulfuric acid | b. | hydrosulfuric acid | d. | sulfurous acid |
|
|
|
28.
|
What is the formula for hydrosulfuric acid?
|
|
|
29.
|
Select the correct formula for sulfur hexafluoride.
|
|
|
30.
|
What is the correct name for the compound CoCl 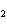 ? a. | cobalt(I) chlorate | c. | cobalt(II) chlorate | b. | cobalt(II) chloride | d. | cobalt(I)
chloride |
|
|
|
31.
|
What is the correct formula for barium chlorate?
a. | Ba(ClO3)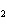 | c. | Ba(ClO)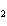 | b. | Ba(ClO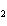 ) )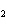 | d. | BaCl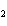 |
|
|
|
32.
|
What is the correct formula for calcium dihydrogen phosphate?
|
|
|
33.
|
Which of the following is the correct name for N 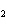 O 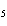 ? a. | nitrous oxide | c. | nitrogen dioxide | b. | dinitrogen pentoxide | d. | nitrate oxide |
|
|
|
34.
|
What is the correct name for Sn 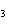 (PO 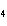 ) 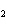 ? a. | tritin diphosphate | c. | tin(III) phosphate | b. | tin(II) phosphate | d. | tin(IV)
phosphate |
|