Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
1.
|
Who was the schoolmaster who studied chemistry and proposed an atomic
theory?
a. | John Dalton | c. | Robert Brown | b. | Jons Berzelius | d. | Dmitri
Mendeleev |
|
|
|
2.
|
Who first recognized that the ratio of the number of atoms that combine is the
same as the ratio of the masses that combine?
a. | Jons Berzelius | c. | John Dalton | b. | Edward Morley | d. | Jon Newlands |
|
|
|
3.
|
According to Dalton's atomic theory, atoms
a. | of different elements combine in simple whole-number ratios to form
compounds. | b. | can be divided into protons, neutrons, and electrons. | c. | of all elements are
identical in size and mass. | d. | can be destroyed in chemical
reactions. |
|
|
|
4.
|
Because most particles fired at metal foil passed straight through, Rutherford
concluded that
a. | atoms were mostly empty space. | c. | electrons formed the
nucleus. | b. | atoms contained no charged particles. | d. | atoms were
indivisible. |
|
|
|
5.
|
Rutherford fired positively charged particles at metal foil and concluded that
most of the mass of an atom was
a. | in the electrons. | c. | evenly spread throughout the atom. | b. | concentrated in the
nucleus. | d. | in rings around
the atom. |
|
|
|
6.
|
A positively charged particle with mass 1.673 ´
10–24 g is a(n)
a. | proton. | c. | electron. | b. | neutron. | d. | positron. |
|
|
|
7.
|
Which part of an atom has a mass approximately equal to 1/2000 of the mass of a
common hydrogen atom?
a. | nucleus | c. | proton | b. | electron | d. | electron cloud |
|
|
|
8.
|
The mass of a neutron is
a. | about the same as that of a proton. | c. | double that of a
proton. | b. | about the same as that of an electron. | d. | double that of an
electron. |
|
|
|
9.
|
The nucleus of most atoms is composed of
a. | tightly packed protons. | c. | tightly packed protons and
neutrons. | b. | tightly packed neutrons. | d. | loosely connected protons and electrons. |
|
|
|
10.
|
Protons have
a. | negative charges. | c. | no charges. | b. | an attraction for neutrons. | d. | no mass. |
|
|
|
11.
|
Most of the volume of an atom is occupied by the
a. | nucleus. | c. | electron cloud. | b. | nuclides. | d. | protons. |
|
|
|
12.
|
The charge on the electron cloud
a. | prevents compounds from forming. | b. | balances the charge on the
nucleus. | c. | attracts electron clouds in other atoms to form compounds. | d. | does not
exist. |
|
|
|
13.
|
The smallest unit of an element that can exist either alone or in combination
with other such particles of the same or different elements is the
a. | electron. | c. | neutron. | b. | proton. | d. | atom. |
|
|
|
14.
|
Atoms of the same element that have different masses are called
a. | moles. | c. | nuclides. | b. | isotopes. | d. | neutrons. |
|
|
|
15.
|
Isotopes of an element contain different numbers of
a. | electrons. | c. | neutrons. | b. | protons. | d. | nuclides. |
|
|
|
16.
|
All isotopes of hydrogen contain
a. | one neutron. | c. | one proton. | b. | two electrons. | d. | two nuclei. |
|
|
|
17.
|
The total number of protons and neutrons in the nucleus of an atom is its
a. | atomic number. | c. | mass number. | b. | Avogadro constant. | d. | number of
neutrons. |
|
|
|
18.
|
As the mass number of the isotopes of an element increases, the number of
protons
a. | decreases. | b. | increases. | c. | remains the
same. | d. | doubles each time the mass number increases. |
|
|
|
19.
|
Atoms of the same element can differ in
a. | chemical properties. | c. | atomic number. | b. | mass number. | d. | number of protons and
electrons. |
|
|
|
20.
|
The abbreviation for atomic mass unit is
|
|
|
21.
|
The average atomic mass of an element depends on both the masses of its isotopes
and each isotope's
a. | atomic number. | c. | relative abundance. | b. | radioactivity. | d. | mass number. |
|
|
|
22.
|
A single atom of an isotope does not have a(n)
a. | relative atomic mass. | c. | mass number. | b. | atomic number. | d. | average atomic
mass. |
|
|
|
|
|
|
23.
|
What is the atomic number for aluminum from the figure above?
|
|
|
24.
|
In the figure above, a neutral atom of silicon contains
a. | 14 electrons. | c. | 16 electrons. | b. | 28.09 electrons. | d. | 38 electrons. |
|
|
|
25.
|
An atom of potassium has 19 protons and 20 neutrons. What is its mass
number?
|
|
|
26.
|
Ag-109 has 62 neutrons. The neutral atom has
a. | 40 electrons. | c. | 53 electrons. | b. | 47 electrons. | d. | 62 electrons. |
|
|
|
27.
|
Carbon-14 (atomic number 6), the radioactive nuclide used in dating fossils,
has
a. | 6 neutrons. | c. | 10 neutrons. | b. | 8 neutrons. | d. | 14 neutrons. |
|
|
|
28.
|
Silicon-30 contains 14 protons. It also contains
a. | 16 electrons. | c. | 30 neutrons. | b. | 16 neutrons. | d. | 44 neutrons. |
|
|
|
29.
|
Neon-22 contains 12 neutrons. It also contains
a. | 12 protons. | c. | 22 electrons. | b. | 22 protons. | d. | 10 protons. |
|
|
|
30.
|
Mendeleev's table was called periodic because the properties of the
elements
a. | showed no pattern. | b. | occurred at repeated intervals called
periods. | c. | occurred at regular time intervals called periods. | d. | were
identical. |
|
|
|
31.
|
What are the elements with atomic numbers from 58 to 71 in the periodic table
called?
a. | the lanthanide elements | c. | the actinide
elements | b. | the noble gases | d. | the alkali metals |
|
|
|
32.
|
Argon, krypton, and xenon are
a. | alkaline earth metals. | c. | actinides. | b. | noble gases. | d. | lanthanides. |
|
|
|
33.
|
In the modern periodic table, elements are ordered according to
a. | decreasing atomic mass. | c. | increasing atomic
number. | b. | Mendeleev's original design. | d. | the date of their
discovery. |
|
|
|
34.
|
The atomic number of lithium, the first element in Group 1, is 3. The atomic
number of the second element in this group is
|
|
|
|
|
|
35.
|
To which group do lithium and potassium belong? Refer to the figure
above.
a. | alkali metals | c. | halogens | b. | transition metals | d. | noble gases |
|
|
|
36.
|
Refer to the figure above. To which group do fluorine and chlorine
belong?
a. | alkaline-earth metals | c. | halogens | b. | transition elements | d. | actinides |
|
|
|
37.
|
What does the number 84 in the name krypton-84 represent?
a. | the atomic number | c. | the sum of the protons and electrons | b. | the mass
number | d. | twice the number of
protons |
|
|
|
38.
|
How many valence electrons are in an atom of phosphorus?
|
|
|
39.
|
How many valence electrons are in an atom of magnesium?
|
|
|
40.
|
What is the charge on the strontium ion?
a. | 2– | c. | 1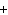 | b. | 1– | d. | 2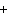 |
|
|
|
41.
|
Which of the following elements does NOT form an ion with a charge of 1 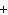 ? a. | fluorine | c. | potassium | b. | hydrogen | d. | sodium |
|
Matching
|
|
|
Match each item with the correct statement below. a. | proton | d. | electron | b. | nucleus | e. | neutron | c. | atom |
|
|
|
42.
|
the smallest particle of an element that retains the properties of that
element
|
|
|
43.
|
a positively charged subatomic particle
|
|
|
44.
|
a negatively charged subatomic particle
|
|
|
45.
|
a subatomic particle with no charge
|
|
|
46.
|
the central part of an atom, containing protons and neutrons
|
|
|
Match each item with the correct statement below. a. | mass number | d. | atomic mass | b. | atomic mass unit | e. | isotope | c. | atomic
number |
|
|
|
47.
|
atoms with the same number of protons, but different numbers of neutrons in the
nucleus of an atom
|
|
|
48.
|
the total number of protons and neutrons in the nucleus of an atom
|
|
|
49.
|
the number of protons in the nucleus of an element
|
|
|
50.
|
the weighted average of the masses of the isotopes of an element
|
|
|
51.
|
one-twelfth the mass of a carbon atom having six protons and six
neutrons
|