Matching
|
|
|
Match each item with the correct statement below. a. | mixture | d. | reactant | b. | product | e. | heterogeneous mixture | c. | phase | f. | vapor |
|
|
|
1.
|
gaseous state of substance that is a liquid or solid at room
temperature
|
|
|
2.
|
a physical blend of two or more components
|
|
|
3.
|
part of a sample having uniform composition and properties
|
|
|
4.
|
not uniform in composition
|
|
|
5.
|
a substance formed in a chemical reaction
|
|
|
6.
|
starting substance in a chemical reaction
|
|
|
Match each item with the correct statement below. a. | distillation | d. | compound | b. | mass | e. | element | c. | chemical
reaction | f. | homogeneous
|
|
|
|
7.
|
amount of matter an object contains
|
|
|
8.
|
describes mixture with a uniform composition
|
|
|
9.
|
a process in which a liquid is boiled to produce a vapor that is condensed
again into a liquid
|
|
|
10.
|
substance that cannot be changed into simpler substances by chemical
means
|
|
|
11.
|
composed of two or more substances chemically combined in a fixed
proportion
|
|
|
12.
|
process in which substances are changed into different substances
|
|
|
Match each item with the correct statement below. a. | product | d. | balanced equation | b. | reactant | e. | skeleton equation | c. | chemical
equation |
|
|
|
13.
|
a chemical equation that does not indicate relative amounts of reactants and
products
|
|
|
14.
|
a new substance formed in a chemical reaction
|
|
|
15.
|
a starting substance in a chemical reaction
|
|
|
16.
|
a concise representation of a chemical reaction
|
|
|
17.
|
an equation in which each side has the same number of atoms of each
element
|
|
|
Match each item with the correct statement below. a. | activity series of metals | c. | combustion
reaction | b. | single-replacement reaction | d. | decomposition reaction |
|
|
|
18.
|
a reaction in which a single compound is broken down into simpler
substances
|
|
|
19.
|
a reaction in which oxygen reacts with another substance, often producing heat
or light
|
|
|
20.
|
a reaction in which the atoms of one element replace the atoms of a second
element in a compound
|
|
|
21.
|
a list of metals in order of decreasing reactivity
|
|
|
Match each of the following with the correct formula a. | CaCO3 | e. | H2O2 | b. | NaClO | f. | C3H8O | c. | MgSO4 | g. | CH3COOH | d. | NH3 |
|
|
|
22.
|
Limestone
|
|
|
23.
|
Bleach
|
|
|
24.
|
Epson Salt
|
|
|
25.
|
Ammonia
|
|
|
26.
|
Hyddrogen Peroxide
|
|
|
27.
|
Isopropyl Alcohol
|
|
|
28.
|
Acetic Acid
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
29.
|
Which field of science studies the composition and structure of matter?
a. | physics | c. | chemistry | b. | biology | d. | geology |
|
|
|
30.
|
The variable that is observed during an experiment is called what type of
variable?
a. | independent | c. | controlling | b. | manipulated | d. | responding |
|
|
|
31.
|
Which of the following is NOT an example of matter?
a. | air | c. | smoke | b. | heat | d. | water vapor |
|
|
|
32.
|
Which of the following is NOT a physical property of water?
a. | It has a boiling point of 100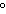 C. C. | b. | It is a colorless
liquid. | c. | It is composed of hydrogen and oxygen. | d. | Sugar dissolves in
it. |
|
|
|
33.
|
Which of the following are considered physical properties of a substance?
a. | color and odor | c. | malleability and hardness | b. | melting and boiling
points | d. | all of the
above |
|
|
|
34.
|
A substance that forms a vapor is generally in what physical state at room
temperature?
a. | solid | c. | gas | b. | liquid | d. | liquid or solid |
|
|
|
35.
|
Which of the following is a physical change?
a. | corrosion | c. | evaporation | b. | explosion | d. | rotting of food |
|
|
|
36.
|
Which of the following is a heterogeneous mixture?
a. | air | c. | steel | b. | salt water | d. | soil |
|
|
|
37.
|
Which of the following is true about homogeneous mixtures?
a. | They are known as solutions. | b. | They consist of two or more
phases. | c. | They have compositions that never vary. | d. | They are always
liquids. |
|
|
|
38.
|
Which of the following is a heterogeneous mixture?
a. | vinegar in water | c. | oil and vinegar | b. | milk | d. | air |
|
|
|
39.
|
An example of a homogeneous mixture is ____.
a. | water | c. | noodle soup | b. | stainless steel | d. | oxygen |
|
|
|
40.
|
Which of the following items is NOT a compound?
a. | baking soda | c. | sucrose | b. | salad dressing | d. | table salt |
|
|
|
41.
|
What is one difference between a mixture and a compound?
a. | A compound consists of more than one phase. | b. | A compound can only
be separated into its components by chemical means. | c. | A mixture can only be separated into its
components by chemical means. | d. | A mixture must be uniform in
composition. |
|
|
|
42.
|
The chemical symbol for iron is ____.
|
|
|
43.
|
Which of the following is NOT a physical change?
a. | grating cheese | c. | fermenting of cheese | b. | melting cheese | d. | mixing two cheeses in a
bowl |
|
|
|
44.
|
A chemical change occurs when a piece of wood ____.
a. | is split | c. | decays | b. | is painted | d. | is cut |
|
|
|
45.
|
Chemical reactions ____.
a. | occur only in living organisms | c. | only occur outside living
organisms | b. | create and destroy atoms | d. | produce new substances |
|
|
|
46.
|
Chemical equations ____.
a. | describe chemical reactions | b. | show how to write chemical
formulas | c. | give directions for naming chemical compounds | d. | describe only
biological changes |
|
|
|
47.
|
In the chemical equation H 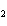 O 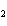 ( aq) ® H 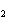 O( l) 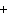 O 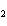 ( g), the 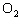 is a ____. a. | catalyst | c. | product | b. | solid | d. | reactant |
|
|
|
48.
|
A catalyst is ____.
a. | the product of a combustion reaction | b. | not used up in a reaction | c. | one of the reactants
in single-replacement reactions | d. | a solid product of a
reaction |
|
|
|
49.
|
Which of the following is the correct skeleton equation for the reaction that
takes place when solid phosphorus combines with oxygen gas to form diphosphorus pentoxide?
|
|
|
50.
|
What are the coefficients that will balance the skeleton equation below?
N 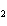 + H 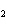 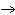 NH 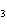 a. | 1, 1, 2 | c. | 3, 1, 2 | b. | 1, 3, 3 | d. | 1, 3, 2 |
|
|
|
51.
|
When the following equation is balanced, what is the coefficient for HCl?
Mg( s) 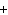 HCl( aq) 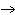 MgCl 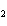 ( aq) 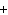 H 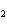 ( g)
|
|
|
52.
|
Chemical equations must be balanced to satisfy ____.
a. | the law of definite proportions | c. | the law of conservation of
mass | b. | the law of multiple proportions | d. | Avogadro’s principle
|
|
|
|
53.
|
When the equation KClO 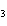 ( s) 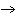 KCl( s) + O 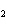 ( g) is balanced, the coefficient of KClO 3 is ____.
|
|
|
54.
|
What are the missing coefficients for the skeleton equation
below? Cr( s) 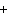 Fe(NO 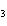 ) 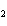 ( aq) 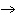 Fe( s) 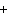 Cr(NO 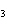 ) 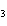 ( aq) a. | 4, 6, 6, 2 | c. | 2, 3, 3, 2 | b. | 2, 3, 2, 3 | d. | 1, 3, 3, 1 |
|
|
|
55.
|
What are the missing coefficients for the skeleton equation below?
Al 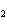 (SO 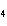 ) 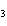 ( aq) 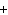
KOH( aq) ® Al(OH) 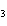 ( aq) 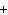 K 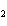 SO 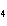 ( aq) a. | 1, 3, 2, 3 | c. | 4, 6, 2, 3 | b. | 2, 12, 4, 6 | d. | 1, 6, 2, 3 |
|
|
|
56.
|
In a combustion reaction, one of the reactants is ____.
a. | hydrogen | c. | oxygen | b. | nitrogen | d. | a metal |
|
|
|
57.
|
In a double-replacement reaction, the ____.
a. | products are always molecular | b. | reactants are two ionic
compounds | c. | reactants are two elements | d. | products are a new element and a new
compound |
|
Short Answer
|
|
|
58.
|
Identify the following and list its Common Name, Family, Genus and
Species 
|
|
|
59.
|
Identify the following and list its Common Name, Family, Genus and
Species 
|
|
|
60.
|
Identify the following and list its Common Name, Family, Genus and
Species 
|
|
|
61.
|
Identify the following and list its Common Name, Family, Genus and
Species 
|
|
|
62.
|
Identify the following and list its Common Name, Family, Genus and Species
What are the symptoms of getting bit by this? 
|
|
|
63.
|
This plant has been used in traditional medicine to relieve asthma symptoms and
as an analgesic during surgery or bonesetting. It is also a powerful hallucinogen and deliriant,
which is used entheogenically for the intense visions it produces. However, the tropane alkaloids
responsible for both the medicinal and hallucinogenic properties are fatally toxic in only slightly
higher amounts than the medicinal dosage, and careless use often results in hospitalizations and
deaths. What is the genus and species of this plant?
|
Essay
|
|
|
64.
|
Define element and compound. Explain the difference between an
element and a compound.
|
|
|
65.
|
Describe some of the physical and chemical changes involved in cooking.
|
|
|
66.
|
State the law of conservation of mass. Then apply the law to this question: What
would be the total mass of the products of a reaction in which 10 grams of water decomposes into the
elements hydrogen and oxygen?
|