1993 #7 Redox- Galvonic Cells
A galvanic cell is constructed using a chromium electrode in a 1.00 molar solution of Cr(NO3)3 and a copper electrode in a 1.00 molar solution of Cu(NO3)2. Both solutions are at 25°C.
(a) Write a balanced net ionic equation for the spontaneous reaction that occurs as the cell operates. Identify the oxidizing agent and the reducing agent.
(b) A partial diagram of the cell is shown below.
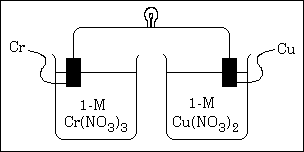
(i) Which metal is the cathode?
(ii) What additional component is necessary to make the cell operate?
(iii) What function does the component in (ii) serve?
(c) How does the potential of this cell change if the concentration of Cr(NO3)3 is changed to 3.00 molar at 25 °C? Explain
1993 #7 Redox- Galvonic Cells Answers
average = 5.1
a) three points
2 Cr + 3 Cu2+ --> 2 Cr3+ + 3 Cu
Cr = reducing agent; Cu2+ = oxidizing agent
b) three points
i) Cu is cathode
ii) salt bridge
iii) transfer of ions or charge but not electrons
c) two points
Nernst equation use
E decreases
Guidelines:
(c) Le Chatelier type argument okay less spontaneous, less formed rxn, more reverse rxn.
If wrong rxn. written, consistency with incorrect rxn. is required. If wrong rxn. is not a redox reaction, points in (bi) and (c) can only be earned if a detailed explanation accompanies. If rxn does not have both an oxidation and a reduction, then no credit can be earned for agents or cathode.
If in part a, reduction and oxidation are correctly labeled, but agents are not addressed, 1 pt can be earned from the "agent" points.