Copyright ©
Houghton Mifflin Company. All rights reserved.
CRS Question, 6–13
QUESTION
(continued)
The
instant cold packs associated with athletic injuries makes use
of the heat change when NH4NO3 dissolves.
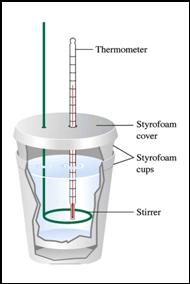
If 8.0 grams of NH4NO3 (molar mass = 80.0) were
placed in 100.0 mL of water such as shown in the constant
pressure calorimeter, and the temperature change was
– 6.0°C, what would you calculate as the DH for dissolving one mole of NH4NO3?
1. –2 500 kJ/mol
2. +2 500 kJ/mol
3. –25 kJ/mol
4. +25 kJ/mol