Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1
|
A measure of the ability of an atom in a chemical compound to attract electrons
is called
A | electron affinity. | C | electronegativity. | B | electron configuration. | D | ionization
potential. |
|
|
|
2
|
The element that has the greatest electronegativity is
A | oxygen. | C | chlorine. | B | sodium. | D | fluorine. |
|
|
|
3
|
When chemical compounds form, valence electrons are those that may be
A | lost only. | C | shared only. | B | gained only. | D | lost, gained, or
shared. |
|
|
|
4
|
For Groups 13 through 18, the number of valence electrons is equal to the group
number
A | plus 1. | C | minus the period number. | B | plus the period
number. | D | minus
10. |
|
|
|
5
|
How many valence electrons are in an atom of phosphorus?
|
|
|
6
|
How many valence electrons does a helium atom have?
|
|
|
7
|
What is the charge on the strontium ion?
A | 2– | C | 1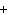 | B | 1– | D | 2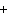 |
|
|
|
8
|
How does oxygen obey the octet rule when reacting to form compounds?
A | It gains electrons. | B | It gives up electrons. | C | It does not change
its number of electrons. | D | Oxygen does not obey the octet
rule. |
|
|
|
9
|
Ionic compounds are normally in which physical state at room temperature?
|
|
|
10
|
Which of the following particles are free to drift in metals?
A | protons | C | neutrons | B | electrons | D | cations |
|
|
|
11
|
An Ionic bond is a bond between ____.
A | a cation and an anion | C | the ions of two different metals | B | valence electrons and
cations | D | the ions of two
different nonmetals |
|
|
|
12
|
A chemical bond results from the mutual attraction of the nuclei of atoms
and
A | electrons. | C | neutrons. | B | protons. | D | dipoles. |
|
|
|
13
|
The Na - F bond in NaF (electronegativity for Na is 0.9; electronegativity for F
is 4.0) is
A | polar covalent. | C | nonpolar covalent. | B | ionic. | D | pure covalent. |
|
|
|
14
|
In which of these compounds is the bond between the atoms NOT a nonpolar
covalent bond?
|
|
|
|
|
|
15
|
What is the Lewis structure for hydrogen chloride, HCl?
|
|
|
16
|
VSEPR theory is a model for predicting
A | the strength of metallic bonds. | C | lattice energy
values. | B | the shape of molecules. | D | ionization energy. |
|
|
|
17
|
Use VSEPR theory to predict the molecular shape of the carbon tetraiodide
molecule, CI4.
A | tetrahedral | C | bent | B | linear | D | trigonal planar |
|
|
|
18
|
Which molecule is linear?
|
|
|
|
|
|
19
|
What is the molecular geometry of this lewis structure (NF3)?
A | square pyramidal | C | trigonal pyramidal | B | tetrahedral | D | linear |
|
|
|
20
|
Which of the following compounds has an ionic
bond?
|
|
|
21
|
Which of the following is the best representation
of the compound rubidium chloride?
|
|
|
22
|
How many valence electrons are in a
Se-2
ion?
|
|
|
23
|
In the Molecular Models Lab, you built the structure for Hydrogen Fluoride. What
is the polarity of that molecule and why?
A | Nonpolar because H and F have the same electronegativities. | C | Polar because F is
more electronegative than H. | B | Polar because H and F are both
nonmetals. | D | Nonpolar because
H is more electronegative than F. |
|
Matching
|
|
|
Match each item with the correct statement below. A | halide ion | E | valence electron | B | octet rule | F | acid base reaction | C | ionic
bond | G | metallic
bond | D | Lewis dot structure | H | bent molecule |
|
|
|
24
|
an electron in the highest occupied energy level of an atom
|
|
|
25
|
Atoms react so as to acquire the stable electron structure of a noble
gas.
|
|
|
26
|
a depiction of valence electrons around the symbol of an element
|
|
|
27
|
the attraction of valence electrons for metal ions
|
|
|
28
|
the force of attraction binding oppositely charged ions together
|
|
|
29
|
Group 7 (or 17) anions
|
|
|
30
|
H2O
|