Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
General Solubility
Guidelines | | 1. Most sodium, potassium, and ammonium compounds are soluble in
water. | | 2. Most nitrates, acetates, and chlorates are soluble.
| | 3. Most chlorides are soluble,
except those of silver, mercury(I), and lead. Lead(II) chloride is soluble in hot
water. | | 4. Most sulfates are soluble, except those of barium, strontium, and
lead. | | 5. Most carbonates, phosphates, and silicates are insoluble, except those of sodium,
potassium, and ammonium. | | 6. Most sulfides are insoluble, except those of calcium, strontium, sodium, potassium, and
ammonium. | |
|
|
|
1.
|
A chemical reaction has NOT occurred if the products have
a. | the same mass as the reactants. | b. | less total bond energy than the
reactants. | c. | more total bond energy than the reactants. | d. | the same chemical
properties as the reactants. |
|
|
|
2.
|
Which observation does NOT indicate that a chemical reaction has
occurred?
a. | formation of a precipitate | c. | evolution of heat and
light | b. | production of a gas | d. | change in total mass of substances |
|
|
|
3.
|
A solid produced by a chemical reaction in solution that separates from the
solution is called
a. | a precipitate. | c. | a molecule. | b. | a reactant. | d. | the mass of the
product. |
|
|
|
4.
|
After the correct formula for a reactant in an equation has been written,
the
a. | subscripts are adjusted to balance the equation. | b. | formula should not
be changed. | c. | same formula must appear as the product. | d. | symbols in the
formula must not appear on the product side of the equation. |
|
|
|
5.
|
What is the small whole number that appears in front of a formula in a chemical
equation?
a. | a subscript | c. | a ratio | b. | a superscript | d. | a coefficient |
|
|
|
6.
|
A chemical equation is balanced when the
a. | coefficients of the reactants equal the coefficients of the
products. | b. | same number of each kind of atom appears in the reactants and in the
products. | c. | products and reactants are the same chemicals. | d. | subscripts of the
reactants equal the subscripts of the products. |
|
|
|
7.
|
Which of the following is a formula equation for the formation of carbon dioxide
from carbon and oxygen?
a. | Carbon plus oxygen yields carbon dioxide. | b. | C + O2
® CO2 | c. | CO2 ® C + O2 | d. | 2C + O ®
CO2 |
|
|
|
8.
|
Which coefficients correctly balance the formula equation
NH4NO2(s)® N2(g) +
H2O(l)?
a. | 1, 2, 2 | c. | 2, 1, 1 | b. | 1, 1, 2 | d. | 2, 2, 2 |
|
|
|
9.
|
After the first steps in writing an equation, the equation is balanced by
a. | adjusting subscripts to the formula(s). | b. | adjusting
coefficients to the smallest whole-number ratio. | c. | changing the products
formed. | d. | making the number of reactants equal to the number of
products. |
|
|
|
10.
|
What is the balanced equation for the combination of sulfur with oxygen to
produce sulfur dioxide?
a. | S(s) + O2(g) ®
SO(g) | c. | 2S(s) + 3O2(g) ®
SO3(s) | b. | S(s) + O2(g) ® SO2(g) | d. | S(s) + 2O2(g) ®
SO42–(aq) |
|
|
|
11.
|
Symbols used in equations, together with the explanations of the symbols, are
shown below. Which set is correct?
a. | (g), grams | c. | (aq), dissolved in water | b. | (l),
liters | d. | (s), soluble |
|
|
|
12.
|
In the chemical equation H 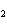 O 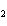 ( aq) ® H 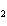 O( l) 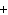 O 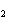 ( g), the 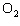 is a ____. a. | catalyst | c. | product | b. | solid | d. | reactant |
|
|
|
13.
|
If you rewrite the following word equation as a balanced chemical equation, what
will the coefficient and symbol for fluorine be? nitrogen trifluoride 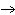 nitrogen 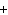 fluorine
|
|
|
14.
|
In every balanced chemical equation, each side of the equation has the same
number of ____.
a. | atoms of each element | c. | moles | b. | molecules | d. | coefficients |
|
|
|
15.
|
What are the missing coefficients for the skeleton equation below?
Al 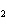 (SO 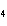 ) 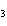 ( aq) 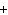
KOH( aq) ® Al(OH) 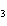 ( aq) 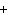 K 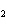 SO 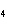 ( aq) a. | 1, 3, 2, 3 | c. | 4, 6, 2, 3 | b. | 2, 12, 4, 6 | d. | 1, 6, 2, 3 |
|
|
|
16.
|
The product of a combination reaction is Ba(OH) 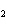 . If
one of the reactants is H 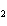 O, what is the other reactant? a. | Ba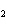 O O | c. | BaH | b. | BaO | d. | BaO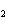 |
|
|
|
17.
|
Which of the following pairs of solutions produces a precipitate when
combined?
a. | KOH and NH4Cl | c. | NH4Cl and
AgNO3 | b. | Na2SO4 and KCl | d. | Fe(NO)3 and
KCl |
|
|
|
18.
|
What is the driving force in the following reaction? Ni(NO 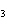 ) 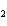 ( aq) 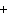 K 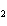 S( aq) 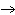 NiS (s) 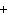 2KNO 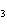 ( aq) a. | Ionic compounds are products. | c. | A precipitate is
formed. | b. | A gas is formed. | d. | Ionic compounds are reactants. |
|
|
|
19.
|
Which of the following is NOT a net ionic equation?
a. | Fe2+(aq) + 2Cl–(aq) +
2OH–(aq) ® Fe(OH)2(s) +
2Cl–(aq) | b. | Ag+(aq) +
Cl–(aq) ® AgCl(s) | c. | Cu2+(aq) + S2–(aq) ® CuS(s) | d. | 3Ca2+(aq) +
2P3–(aq) ®
Ca3P2(s) |
|
|
|
20.
|
A double-replacement reaction takes place when aqueous Na 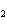 CO 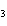 reacts with aqueous Sn(NO 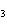 ) 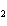 . You would expect one of the products
of this reaction to be ____.
|
|
|
21.
|
The equation 2C 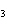 H 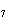 OH 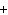 9O 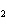 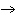 6CO 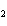 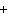 8H 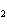 O is an example of which type of reaction? a. | single-replacement reaction | c. | decomposition
reaction | b. | double-replacement reaction | d. | combustion reaction |
|
|
|
22.
|
When solutions of two ionic compounds are combined and a solid forms, the
process is called
a. | dissociation. | c. | precipitation. | b. | solvation. | d. | hydration. |
|
|
|
23.
|
What is the net ionic equation for the precipitation reaction between copper(II)
chloride and sodium hydroxide?
a. | Na+(aq) + Cl–(aq) ® NaCl(s) | c. | Cu2+(aq) +
2OH–(aq) ®
Cu(OH)2(s) | b. | Cu2+(aq) +
2Cl–(aq) + 2Na+(aq) + 2OH–(aq)
® Cu(OH)2(s) + 2NaCl(s) | d. | Cu2+(aq) +
2OH–(aq) + 2Cl–(aq) ®
Cu(OH)2(s) + 2Cl–(aq) |
|
|
|
24.
|
Which of the following dissociates in water?
a. | AgCl | c. | MgCO3 | b. | NaCl | d. | BaSO4 |
|
|
|
25.
|
What is the spectator ion in the equation
2Na+(aq) +
S2–(aq)+ Zn2+(aq) +
2NO3–(aq)® 2Na+(aq)+
2NO3-(aq) + ZnS(s)?
a. | S2–(aq) | c. | Na+(aq) | b. | ZnS(s) | d. | none of these |
|
|
|
26.
|
In a double-replacement reaction, the ____.
a. | products are a new element and a new compound | c. | reactants are usually two ionic
compounds | b. | reactants are two elements | d. | products are always carbon dioxide and water |
|
|
|
27.
|
In a combustion reaction, one of the reactants is always ____.
a. | a metal | c. | hydrogen | b. | oxygen | d. | nitrogen |
|
|
|
28.
|
The type of reaction that takes place when one element reacts with a compound to
form a new compound and a different element is a ____.
a. | single-replacement reaction | c. | decomposition
reaction | b. | double-replacement reaction | d. | combination/synthesis reaction |
|
Matching
|
|
|
Match each equation with its correct type a. | synthesis | c. | single replacement | b. | acid-base reaction/double
replacement | d. | precipitation
reaction/double replacement |
|
|
|
29.
|
H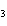 PO PO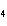 (aq)
(aq)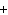 3KOH(aq) 3KOH(aq) 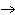 K K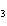 PO PO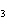 (aq) (aq)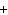 3H 3H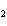 O(l) O(l)
|
|
|
30.
|
2H2(g) + O2(g) 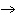 2H2O(g) 2H2O(g)
|
|
|
31.
|
Ba(NO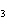 ) )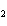 (aq) (aq) 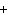 K K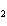 SO4(aq) SO4(aq) 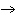 BaSO4(s)
BaSO4(s) 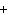 2KNO
2KNO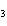 (aq) (aq)
|
|
|
32.
|
Mg(s) 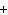 2HCl(aq) 2HCl(aq) 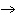 MgCl MgCl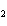 (aq) (aq) 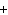 H H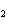 (g) (g)
|